Powder: -20°C for 3 years | In solvent: -80°C for 1 year


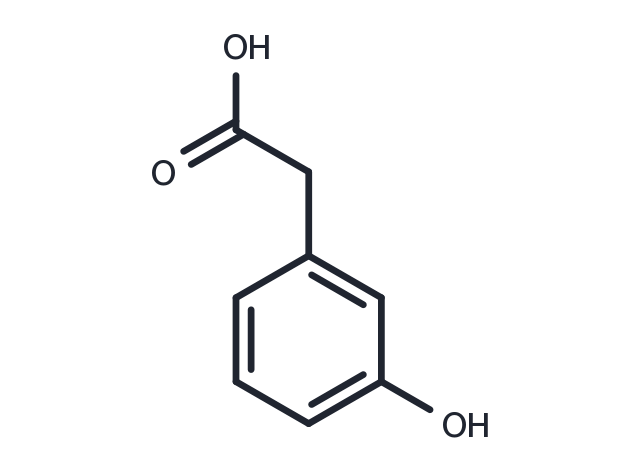
3-Hydroxyphenylacetic acid is a rutin metabolite and an antioxidant. It has a protective biological activity in human. It is a substrate of enzyme 4-hydroxyphenylacetate 3-monooxygenase [EC 1.14.13.3] in the pathway tyrosine metabolism. 3-Hydroxyphenylacetic acid is found to be associated with phenylketonuria, which is an inborn error of metabolism.
| Pack Size | Availability | Price/USD | Quantity |
|---|---|---|---|
| 100 mg | In stock | $ 42.00 | |
| 1 mL * 10 mM (in DMSO) | In stock | $ 46.00 |


| Description | 3-Hydroxyphenylacetic acid is a rutin metabolite and an antioxidant. It has a protective biological activity in human. It is a substrate of enzyme 4-hydroxyphenylacetate 3-monooxygenase [EC 1.14.13.3] in the pathway tyrosine metabolism. 3-Hydroxyphenylacetic acid is found to be associated with phenylketonuria, which is an inborn error of metabolism. |
| Molecular Weight | 152.15 |
| Formula | C8H8O3 |
| CAS No. | 621-37-4 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
DMSO: 55 mg/mL (361.49 mM)
You can also refer to dose conversion for different animals. More
bottom
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc.
3-Hydroxyphenylacetic acid 621-37-4 Metabolism Others Endogenous Metabolite 3Hydroxyphenylacetic acid 3 Hydroxyphenylacetic acid inhibit Inhibitor inhibitor
