- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Influenza A H1N1 (A/Puerto Rico/8/34/Mount Sinai) Non-structural/NS1 Protein (His)
The NS1 Influenza protein is created by the internal protein-encoding, linear negative-sense, single-stranded RNA, NS gene segment and which also codes for the nuclear export protein or NEP, formerly referred to as the NS2 protein, which mediates the export of vRNPs. The non-structural (NS1) protein is found in Influenzavirus A, Influenzavirus B, and Influenzavirus C. The non-structural (NS1) protein of the highly pathogenic avian H5N1 viruses circulating in poultry and waterfowl in Southeast Asia is currently believed to be responsible for the enhanced virulence of the strain. The Non-structural (NS1) protein of influenza A virus is a non-essential virulence factor that has multiple accessory functions during viral infection. The major role ascribed to NS1 has been its inhibition of host immune responses, especially the limitation of both interferon (IFN) production and the antiviral effects of IFN-induced proteins, such as dsRNA-dependent protein kinase R (PKR) and 2'5'-oligoadenylate synthetase (OAS)/RNase L. Non-structural (NS1) protein is a non-structural protein of the influenza A virus, which could only be expressed when cells are infected. The effect of NS1 protein on the host cell is still not clear. Not only could NS1 remarkably affect metabolism, but it could also slow down cell proliferation by blocking the cell cycle. Non-structural (NS1) protein may lead to the development of novel antiviral drugs, and the use of oncolytic influenza A viruses as potential anti-cancer agents.

Influenza A H1N1 (A/Puerto Rico/8/34/Mount Sinai) Non-structural/NS1 Protein (His)
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 μg | $517 | In Stock | |
| 200 μg | $883 | 7-10 days | |
| 500 μg | $1,780 | 7-10 days |
Product Information
| Biological Activity | Activity testing is in progress. It is theoretically active, but we cannot guarantee it. If you require protein activity, we recommend choosing the eukaryotic expression version first. |
| Description | The NS1 Influenza protein is created by the internal protein-encoding, linear negative-sense, single-stranded RNA, NS gene segment and which also codes for the nuclear export protein or NEP, formerly referred to as the NS2 protein, which mediates the export of vRNPs. The non-structural (NS1) protein is found in Influenzavirus A, Influenzavirus B, and Influenzavirus C. The non-structural (NS1) protein of the highly pathogenic avian H5N1 viruses circulating in poultry and waterfowl in Southeast Asia is currently believed to be responsible for the enhanced virulence of the strain. The Non-structural (NS1) protein of influenza A virus is a non-essential virulence factor that has multiple accessory functions during viral infection. The major role ascribed to NS1 has been its inhibition of host immune responses, especially the limitation of both interferon (IFN) production and the antiviral effects of IFN-induced proteins, such as dsRNA-dependent protein kinase R (PKR) and 2'5'-oligoadenylate synthetase (OAS)/RNase L. Non-structural (NS1) protein is a non-structural protein of the influenza A virus, which could only be expressed when cells are infected. The effect of NS1 protein on the host cell is still not clear. Not only could NS1 remarkably affect metabolism, but it could also slow down cell proliferation by blocking the cell cycle. Non-structural (NS1) protein may lead to the development of novel antiviral drugs, and the use of oncolytic influenza A viruses as potential anti-cancer agents. |
| Species | H1N1 |
| Expression System | E. coli |
| Tag | N-His |
| Accession Number | C8XP22 |
| Synonyms | NS1 Protein |
| Construction | A DNA sequence encoding the influenza A H1N1 Virus (A/Puerto Rico/8/34/Mount Sinai) NS1 protein (C8XP22) (Asp 2-Val 230) was expressed, with a polyhistidine tag at the N-terminus. Predicted N terminal: Met |
| Protein Purity | > 90 % as determined by SDS-PAGE 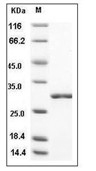 |
| Molecular Weight | 27.2 kDa (predicted); 29 kDa (reducing conditions) |
| Endotoxin | Please contact us for more information. |
| Formulation | Lyophilized from a solution filtered through a 0.22 μm filter, containing PBS, 5% glycerol, pH 8.0.Typically, a mixture containing 5% to 8% trehalose, mannitol, and 0.01% Tween 80 is incorporated as a protective agent before lyophilization. |
| Reconstitution | A Certificate of Analysis (CoA) containing reconstitution instructions is included with the products. Please refer to the CoA for detailed information. |
| Stability & Storage | It is recommended to store recombinant proteins at -20°C to -80°C for future use. Lyophilized powders can be stably stored for over 12 months, while liquid products can be stored for 6-12 months at -80°C. For reconstituted protein solutions, the solution can be stored at -20°C to -80°C for at least 3 months. Please avoid multiple freeze-thaw cycles and store products in aliquots. |
| Shipping | In general, Lyophilized powders are shipping with blue ice. |
| Research Background | The NS1 Influenza protein is created by the internal protein-encoding, linear negative-sense, single-stranded RNA, NS gene segment and which also codes for the nuclear export protein or NEP, formerly referred to as the NS2 protein, which mediates the export of vRNPs. The non-structural (NS1) protein is found in Influenzavirus A, Influenzavirus B, and Influenzavirus C. The non-structural (NS1) protein of the highly pathogenic avian H5N1 viruses circulating in poultry and waterfowl in Southeast Asia is currently believed to be responsible for the enhanced virulence of the strain. The Non-structural (NS1) protein of influenza A virus is a non-essential virulence factor that has multiple accessory functions during viral infection. The major role ascribed to NS1 has been its inhibition of host immune responses, especially the limitation of both interferon (IFN) production and the antiviral effects of IFN-induced proteins, such as dsRNA-dependent protein kinase R (PKR) and 2'5'-oligoadenylate synthetase (OAS)/RNase L. Non-structural (NS1) protein is a non-structural protein of the influenza A virus, which could only be expressed when cells are infected. The effect of NS1 protein on the host cell is still not clear. Not only could NS1 remarkably affect metabolism, but it could also slow down cell proliferation by blocking the cell cycle. Non-structural (NS1) protein may lead to the development of novel antiviral drugs, and the use of oncolytic influenza A viruses as potential anti-cancer agents. |
Dose Conversion
Calculator
Tech Support

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.


