Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

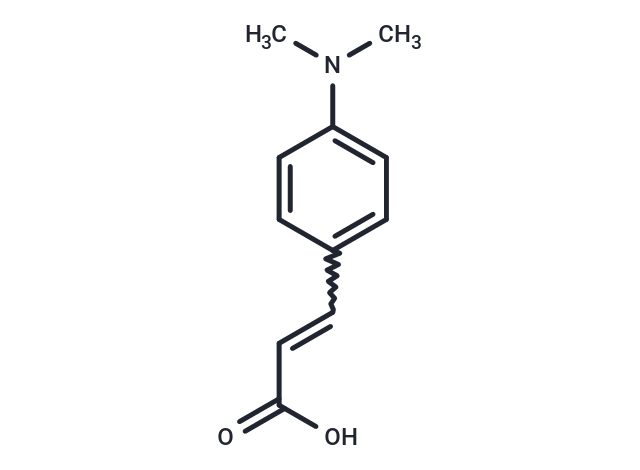
4-(Dimethylamino)cinnamic acid (DMACA) could be as charge transfer (ICT) probe.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 mg | $76 | 7-10 days |
| Description | 4-(Dimethylamino)cinnamic acid (DMACA) could be as charge transfer (ICT) probe. |
| In vitro | Cinnamic acid (CA) derivatives are known to possess broad therapeutic applications including anti-tumor activity. The present study was designed to determine the underlying mechanism and thermodynamic parameters for the binding of two CA based intramolecular charge transfer (ICT) fluorescent probes, namely, 4-(dimethylamino) cinnamic acid (DMACA) and trans-ethyl p-(dimethylamino) cinnamate (EDAC), with albumins by fluorescence spectroscopy. METHODS AND RESULTS:Stern-Volmer analysis of the tryptophan fluorescence quenching data in presence of the added ligand reveals fluorescence quenching constant (κ(q)), Stern-Volmer constant (K(SV)) and also the ligand-protein association constant (K(a)). The thermodynamic parameters like enthalpy (ΔH) and entropy (ΔS) change corresponding to the ligand binding process were also estimated. The results show that the ligands bind into the sub-domain IIA of the proteins in 1:1 stoichiometry with an apparent binding constant value in the range of 10(4) dm(3) mol(-1). CONCLUSIONS:In both the cases, the spontaneous ligand binding to the proteins occur through entropy driven mechanism, although the interaction of DMACA is relatively stronger in comparison with EDAC. The temperature dependence of the binding constant indicates the induced change in protein secondary structure. |
| Molecular Weight | 191.23 |
| Formula | C11H13NO2 |
| Cas No. | 1552-96-1 |
| Relative Density. | 1.31g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.