- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
AMK (hydrochloride)
AMK is an active metabolite of the neurohormone melatonin .1,2,3,4It is formed from melatoninviathe metabolic intermediate AFMK that is then deformylated by catalase or formamidase.5,6AMK scavenges singlet oxygenin vitrowhen used at a concentration of 200 μM.1It inhibits the epinephrine- and arachidonic acid-induced production of prostaglandin E2and PGD2in ovine seminal vesicle microsomes in a concentration- and time-dependent manner, as well as LPS-induced increases in COX-2 levels in RAW 264.7 macrophages when used at a concentration of 500 μM.2,3AMK (20 mg/kg) decreases MPTP-induced increases in lipid peroxidation in the cytosol and mitochondria from substantia nigra and striatum in a mouse model of MPTP-induced Parkinson's disease.4
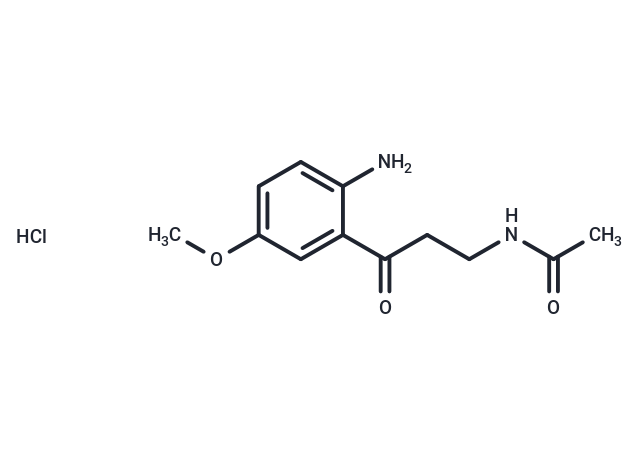
AMK (hydrochloride)
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | Inquiry | 35 days | |
| 5 mg | Inquiry | 35 days |
Product Introduction
| Description | AMK is an active metabolite of the neurohormone melatonin .1,2,3,4It is formed from melatoninviathe metabolic intermediate AFMK that is then deformylated by catalase or formamidase.5,6AMK scavenges singlet oxygenin vitrowhen used at a concentration of 200 μM.1It inhibits the epinephrine- and arachidonic acid-induced production of prostaglandin E2and PGD2in ovine seminal vesicle microsomes in a concentration- and time-dependent manner, as well as LPS-induced increases in COX-2 levels in RAW 264.7 macrophages when used at a concentration of 500 μM.2,3AMK (20 mg/kg) decreases MPTP-induced increases in lipid peroxidation in the cytosol and mitochondria from substantia nigra and striatum in a mouse model of MPTP-induced Parkinson's disease.4 1.Schaefer, M., and Hardeland, R.The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavengerJ. Pineal Res.46(1)49-52(2009) 2.Kelly, R.W., Amato, F., and Seamark, R.F.N-acetyl-5-methoxy kynurenamine, a brain metabolite of melatonin, is a potent inhibitor of prostaglandin biosynthesisBiochem. Biophys. Res. Commun.121(1)372-379(1984) 3.Mayo, J.C., Sainz, R.M., Tan, D.-X., et al.Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophagesJ. Neuroimmunol.165(1-2)139-149(2005) 4.Tapias, V., Escames, G., López, L.C., et al.Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian miceJ. Neurosci. Res.87(13)3002-3010(2009) 5.Tan, D.-X., Manchester, L.C., Reiter, R.J., et al.Melatonin directly scavenges hydrogen peroxide: A potentially new metabolic pathway of melatonin biotransformationFree Radic. Biol. Med.29(11)1177-1185(2000) 6.Hirata, F., Hayaishi, O., Tokuyama, T., et al.In vitro and in vivo formation of two new metabolites of melatoninJ. Biol. Chem.249(4)1311-1313(1974) |
| Molecular Weight | 272.73 |
| Formula | C12H17ClN2O3 |
| Cas No. | 1215711-91-3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | Methanol: Slightly soluble H2O: Slightly soluble |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support
Keywords

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.




