- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Olmutinib hydrochloride
Olmutinib is a novel third-generation epidermal growth factor receptor (EGFR) mutation-specific tyrosine kinase inhibitor, used in the treatment of T790M mutation positive non-small cell lung cancer. Olmutinib covalently binds a cysteine residue near the kinase domain of mutant EGFRs to prevent phosphorylation of the receptor. EGFRs are frequently over-expressed in lung cancer and contribute to activation of the phosphoinositide 3-kinase and mitogen-activated protein kinase pathways which both promote cell survival and proliferation. By inhibiting EGFR activation, Olmutinib attenuates the activation of these tumor-promoting pathways. In the first phase I/II clinical study of Osimertinib, 800 mg/ day was chosen as the dose for subsequent studies, and the dose-limiting toxicity and maximum tolerated dose was not reached. Olmutinib received breakthrough therapy designation in the United States in December 2015 and was approved for use in Korea in May 2016.
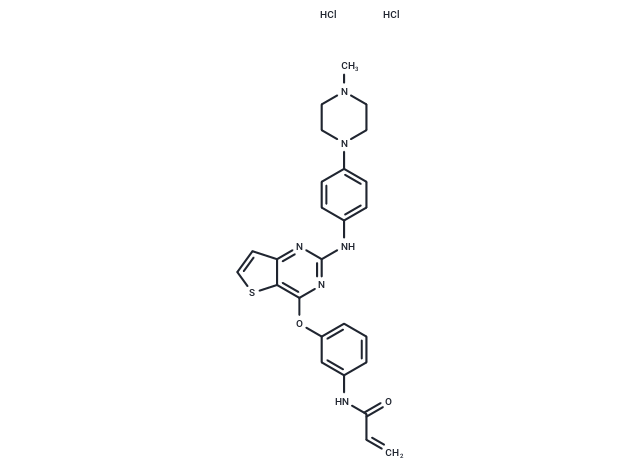
Olmutinib hydrochloride
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $1,520 | 1-2 weeks | |
| 50 mg | $1,980 | 1-2 weeks | |
| 100 mg | $2,500 | 1-2 weeks |
Product Introduction
| Description | Olmutinib is a novel third-generation epidermal growth factor receptor (EGFR) mutation-specific tyrosine kinase inhibitor, used in the treatment of T790M mutation positive non-small cell lung cancer. Olmutinib covalently binds a cysteine residue near the kinase domain of mutant EGFRs to prevent phosphorylation of the receptor. EGFRs are frequently over-expressed in lung cancer and contribute to activation of the phosphoinositide 3-kinase and mitogen-activated protein kinase pathways which both promote cell survival and proliferation. By inhibiting EGFR activation, Olmutinib attenuates the activation of these tumor-promoting pathways. In the first phase I/II clinical study of Osimertinib, 800 mg/ day was chosen as the dose for subsequent studies, and the dose-limiting toxicity and maximum tolerated dose was not reached. Olmutinib received breakthrough therapy designation in the United States in December 2015 and was approved for use in Korea in May 2016. |
| Molecular Weight | 559.51 |
| Formula | C26H28Cl2N6O2S |
| Cas No. | 1842366-97-5 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.




