- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
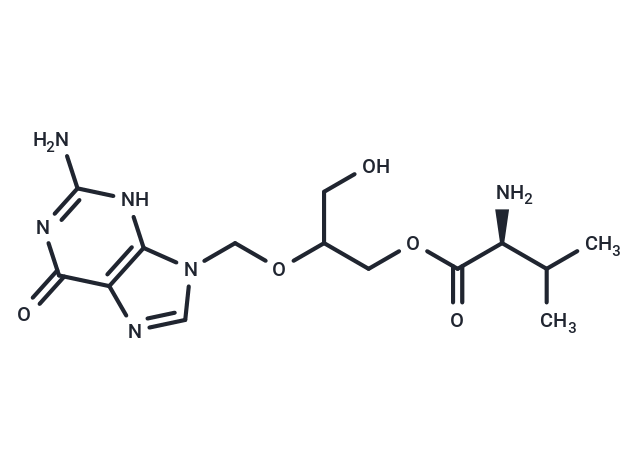
Valganciclovir
Valganciclovir, the L-valyl ester of ganciclovir, is a prodrug effectively converted to ganciclovir in the body, serving as an antiviral medication for cytomegalovirus (CMV) infections. In vitro studies demonstrate that valganciclovir inhibits glycylsarcosine transport mediated by PEPT1 and PEPT2 in Caco-2 and SKPT cells, respectively, with competitive inhibition K(i) values of 1.68±0.30 mM for PEPT1 and 0.043±0.005 mM for PEPT2. Clinical trials indicate valganciclovir's non-inferior efficacy to ganciclovir in pre-emptive therapy for CMV, exhibiting no significant difference in viral clearance rates or toxicity levels between the treatments. Specifically, 89.5% of patients treated with valganciclovir and 83% treated with ganciclovir achieved viral clearance at 28 days (P=0.030 for non-inferiority). Additionally, valganciclovir prophylaxis at 450 mg twice daily prevented CMV reactivation in patients on an alemtuzumab-containing regimen, with none of the 20 patients experiencing reactivation (P=0.004).
Valganciclovir
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $1,520 | 1-2 weeks | |
| 50 mg | $1,980 | 1-2 weeks | |
| 100 mg | $2,500 | 1-2 weeks |
Product Introduction
| Description | Valganciclovir, the L-valyl ester of ganciclovir, is a prodrug effectively converted to ganciclovir in the body, serving as an antiviral medication for cytomegalovirus (CMV) infections. In vitro studies demonstrate that valganciclovir inhibits glycylsarcosine transport mediated by PEPT1 and PEPT2 in Caco-2 and SKPT cells, respectively, with competitive inhibition K(i) values of 1.68±0.30 mM for PEPT1 and 0.043±0.005 mM for PEPT2. Clinical trials indicate valganciclovir's non-inferior efficacy to ganciclovir in pre-emptive therapy for CMV, exhibiting no significant difference in viral clearance rates or toxicity levels between the treatments. Specifically, 89.5% of patients treated with valganciclovir and 83% treated with ganciclovir achieved viral clearance at 28 days (P=0.030 for non-inferiority). Additionally, valganciclovir prophylaxis at 450 mg twice daily prevented CMV reactivation in patients on an alemtuzumab-containing regimen, with none of the 20 patients experiencing reactivation (P=0.004). |
| Molecular Weight | 354.36 |
| Formula | C14H22N6O5 |
| Cas No. | 175865-60-8 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.




