Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

GM-CSF/CSF2 Protein, Human, Recombinant is expressed in HEK293 mammalian cells. The predicted molecular weight is 14.5 kDa and the accession number is P04141.

| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 20 μg | $136 | In Stock | |
| 100 μg | $498 | 7-10 days | |
| 200 μg | $789 | 7-10 days | |
| 500 μg | $2,250 | 7-10 days |
| Biological Activity | Measured in a cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is typically 0.06-0.3 ng/mL. 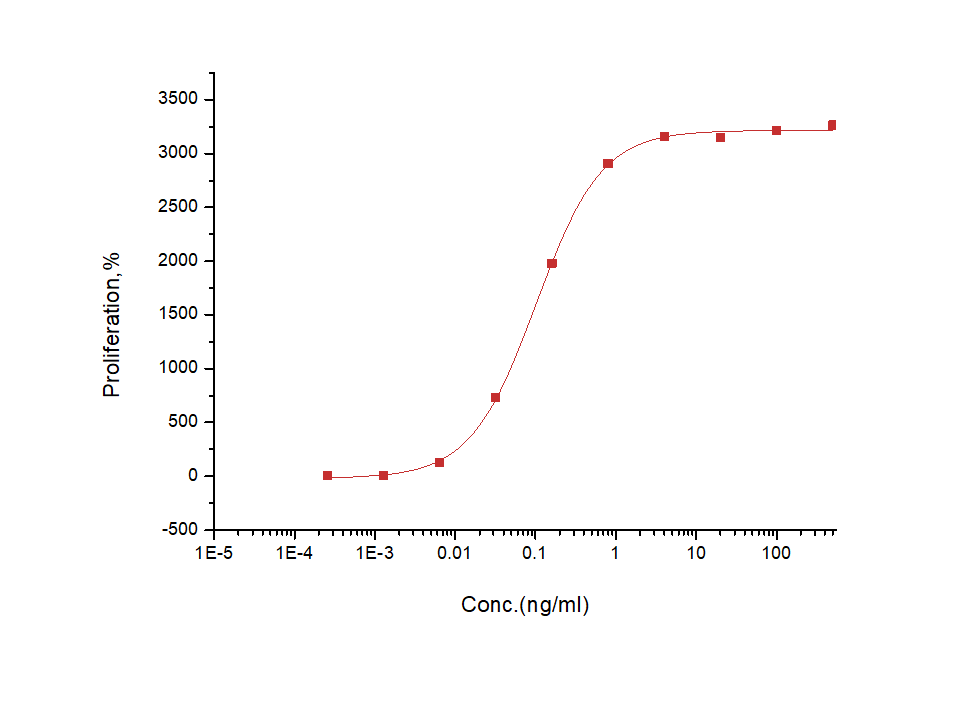 |
| Description | GM-CSF/CSF2 Protein, Human, Recombinant is expressed in HEK293 mammalian cells. The predicted molecular weight is 14.5 kDa and the accession number is P04141. |
| Species | Human |
| Expression System | HEK293 Cells |
| Tag | Tag Free |
| Accession Number | P04141 |
| Synonyms | GM-CSF,GMCSF,CSF2,colony stimulating factor 2 (granulocyte-macrophage) |
| Construction | A DNA sequence encoding human GMCSF (NP_000749.2) (Met1-Glu144) was expressed. Predicted N terminal: Ala 18 |
| Protein Purity | ≥ 95 % as determined by SDS-PAGE. ≥ 95 % as determined by SEC-HPLC. 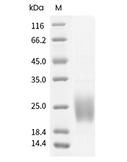 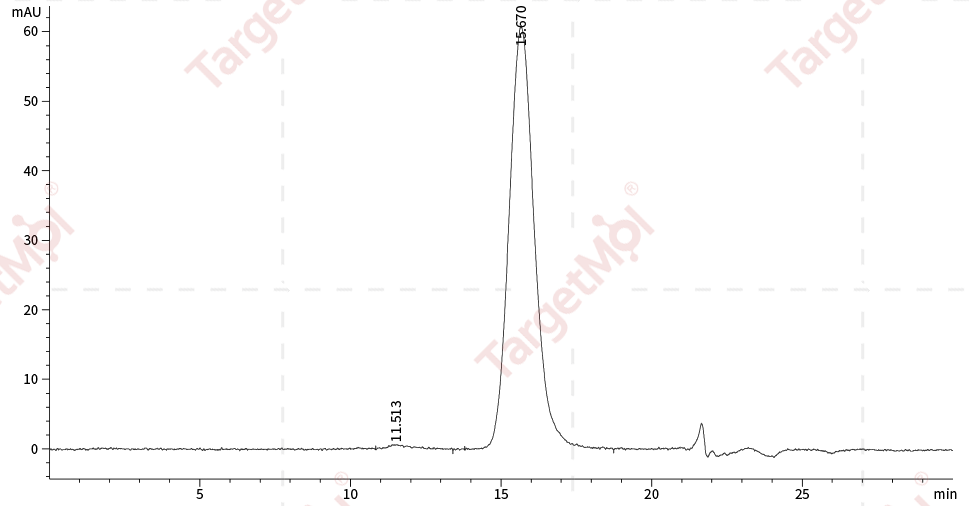 |
| Molecular Weight | 14.5 kDa (predicted); 19-29 kDa (reducing condition, due to glycosylation) |
| Endotoxin | < 10 EU/mg of the protein. |
| Formulation | Lyophilized from a solution filtered through a 0.22 μm filter, containing PBS, pH 7.4. Typically, a mixture containing 5% to 8% trehalose, mannitol, and 0.01% Tween 80 is incorporated as a protective agent before lyophilization. |
| Reconstitution | A Certificate of Analysis (CoA) containing reconstitution instructions is included with the products. Please refer to the CoA for detailed information. |
| Stability & Storage | It is recommended to store recombinant proteins at -20°C to -80°C for future use. Lyophilized powders can be stably stored for over 12 months, while liquid products can be stored for 6-12 months at -80°C. For reconstituted protein solutions, the solution can be stored at -20°C to -80°C for at least 3 months. Please avoid multiple freeze-thaw cycles and store products in aliquots. |
| Shipping | In general, Lyophilized powders are shipping with blue ice. |
| Research Background | Granulocyte-macrophage colony-stimulating factor (GM-CSF) is one of an array of cytokines with pivotal roles in embryo implantation and subsequent development. Several cell lineages in the reproductive tract and gestational tissues synthesise GM-CSF under direction by ovarian steroid hormones and signalling agents originating in male seminal fluid and the conceptus. The pre-implantation embryo, invading placental trophoblast cells and the abundant populations of leukocytes controlling maternal immune tolerance are all subject to GM-CSF regulation. GM-CSF stimulates the differentiation of hematopoietic progenitors to monocytes and neutrophils, and reduces the risk for febrile neutropenia in cancer patients. GM-CSF also has been shown to induce the differentiation of myeloid dendritic cells (DCs) that promote the development of T-helper type 1 (cellular) immune responses in cognate T cells. The active form of the protein is found extracellularly as a homodimer, and the encoding gene is localized to a related gene cluster at chromosome region 5q31 which is known to be associated with 5q-syndrome and acute myelogenous leukemia. As a part of the immune/inflammatory cascade, GM-CSF promotes Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity, and thus worthy of consideration for therapeutic target. GM-CSF has been utilized in the clinical management of multiple disease processes. Most recently, GM-CSF has been incorporated into the treatment of malignancies as a sole therapy, as well as a vaccine adjuvant. While the benefits of GM-CSF in this arena have been promising, recent reports have suggested the potential for GM-CSF to induce immune suppression and, thus, negatively impact outcomes in the management of cancer patients. GM-CSF deficiency in pregnancy adversely impacts fetal and placental development, as well as progeny viability and growth after birth, highlighting this cytokine as a central maternal determinant of pregnancy outcome with clinical relevance in human fertility.Cancer ImmunotherapyImmune CheckpointImmunotherapyTargeted Therapy |

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.