- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
16α-Hydroxyestrone
The naturally-occurring estrogens are estrone , estradiol , and estriol . 16α-hydroxy Estrone (16α-OHE1) is a hydroxylated metabolite of E1 as well as an interconversion product with E2. E1 is 16α-hydroxylated by cytochrome P450 (CYP) isoforms, including CYP1A1, CYP3A5, CYP3A4, and CYP3A7, with CYP3A5 being breast-specific. 16α-OHE1 is sulphatized or glucuronidated before excretion. It is increased in rheumatoid arthritis and decreased by physical activity. Unlike the parent estrogens and other hydroxylated metabolites of E1, 16α-OHE1 binds covalently and persistently activates estrogen receptors. In addition, this metabolite increases cell proliferation and does not suppress TNF-α secretion, whereas other estrogen metabolites are not pro-proliferative and have marked effects on TNF-α secretion. The levels of 16α-OHE1 are increased in some forms of hormone therapy. Because hormone therapy increases breast cancer risk, 16α-OHE1 has been implicated as a risk factor for breast cancer, although supportive data remains elusive.
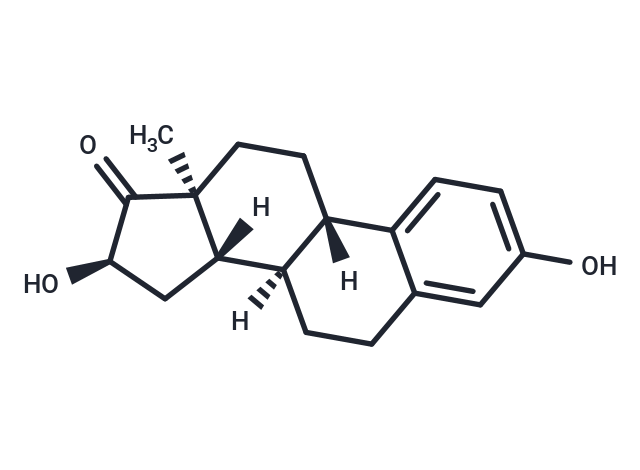
16α-Hydroxyestrone
Product information is being updated, if you want to purchase, please click the bulk custom button.
Product Introduction
| Description | The naturally-occurring estrogens are estrone , estradiol , and estriol . 16α-hydroxy Estrone (16α-OHE1) is a hydroxylated metabolite of E1 as well as an interconversion product with E2. E1 is 16α-hydroxylated by cytochrome P450 (CYP) isoforms, including CYP1A1, CYP3A5, CYP3A4, and CYP3A7, with CYP3A5 being breast-specific. 16α-OHE1 is sulphatized or glucuronidated before excretion. It is increased in rheumatoid arthritis and decreased by physical activity. Unlike the parent estrogens and other hydroxylated metabolites of E1, 16α-OHE1 binds covalently and persistently activates estrogen receptors. In addition, this metabolite increases cell proliferation and does not suppress TNF-α secretion, whereas other estrogen metabolites are not pro-proliferative and have marked effects on TNF-α secretion. The levels of 16α-OHE1 are increased in some forms of hormone therapy. Because hormone therapy increases breast cancer risk, 16α-OHE1 has been implicated as a risk factor for breast cancer, although supportive data remains elusive. |
| Molecular Weight | 286.37 |
| Formula | C18H22O3 |
| Cas No. | 566-76-7 |
| Relative Density. | 1.249 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO: 20 mg/mL (69.84 mM), Sonication is recommended. DMF: 30 mg/mL (104.76 mM), Sonication is recommended. DMF:PBS (pH 7.2) (1:5): 0.15 mg/mL (0.52 mM), Sonication is recommended. |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support
Keywords

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



