- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Piperazine 2HCl
Piperazine (2HCl) is gamma-aminobutyric acid (GABA) agonists and its major effects appear to be on the central nervous system. Piperazine was the anthelmintic with the greatest number of reports of toxicoses and suspected toxicoses in cats. Piperazine neurotoxicity in cats and dogs usually was manifested by muscle tremors, ataxia, and/or behavioral disturbances within 24 hours after estimated daily dose(s) between 20 and 110 mg/kg[1]. For di-substituted derivatives, ciprofloxacin was selected and hybrids were synthesized via substitution at piperazinyl-N4. The reaction of piperazinyl-NH of ciprofloxacin with selected drugs resulted in pronounced growth inhibition of standard as well as resistant bacterial strains[2]. The parent piperazine 6 was found to exhibit a reasonable activity toward the HeLa and MDA MB 231 tumor cell lines (IC50= 9.2 and 8.4 μΜ, respectively)[3]. Piperazine adipate (10 mM) causes mortality of A. galli and H. gallinae after a maximum of 30 min exposure, inhibits malate oxidation by 78%, and inhibits aldolase activity in both parasites. Piperazine adipate (10 mM) also inhibits cholinesterase activity by 96% in Ascaridia galli (A. galli) and 93% in Heterakis gallinae (H. gallinae). Piperazine adipate inhibits oxaloacetate reduction by 26% and 55% in A. galli and H. gallinae, resepctively[4].
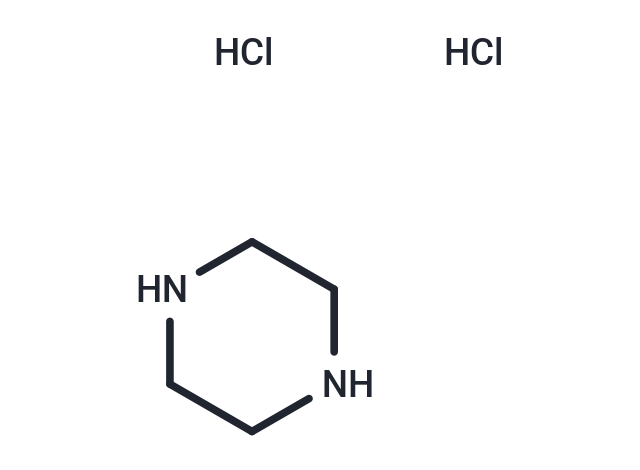
Piperazine 2HCl
Product information is being updated, if you want to purchase, please click the bulk custom button.
Product Introduction
| Description | Piperazine (2HCl) is gamma-aminobutyric acid (GABA) agonists and its major effects appear to be on the central nervous system. Piperazine was the anthelmintic with the greatest number of reports of toxicoses and suspected toxicoses in cats. Piperazine neurotoxicity in cats and dogs usually was manifested by muscle tremors, ataxia, and/or behavioral disturbances within 24 hours after estimated daily dose(s) between 20 and 110 mg/kg[1]. For di-substituted derivatives, ciprofloxacin was selected and hybrids were synthesized via substitution at piperazinyl-N4. The reaction of piperazinyl-NH of ciprofloxacin with selected drugs resulted in pronounced growth inhibition of standard as well as resistant bacterial strains[2]. The parent piperazine 6 was found to exhibit a reasonable activity toward the HeLa and MDA MB 231 tumor cell lines (IC50= 9.2 and 8.4 μΜ, respectively)[3]. Piperazine adipate (10 mM) causes mortality of A. galli and H. gallinae after a maximum of 30 min exposure, inhibits malate oxidation by 78%, and inhibits aldolase activity in both parasites. Piperazine adipate (10 mM) also inhibits cholinesterase activity by 96% in Ascaridia galli (A. galli) and 93% in Heterakis gallinae (H. gallinae). Piperazine adipate inhibits oxaloacetate reduction by 26% and 55% in A. galli and H. gallinae, resepctively[4]. |
| Molecular Weight | 159.05 |
| Formula | C4H12Cl2N2 |
| Cas No. | 142-64-3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



