- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
(-)-Cevimeline hydrochloride hemihydrate
Cevimeline hydrochloride hemihydrate ((-)-SNI-2011), a novel muscarinic receptor agonist, is being explored as a potential treatment for xerostomia in Sjogren's syndrome, exhibiting an IC50 value indicative of its affinity for mAChR. This compound's pharmacological effects on the gastrointestinal, urinary, and reproductive systems, alongside its impact on various tissues, were thoroughly examined in species including mice, rats, guinea pigs, rabbits, and dogs. The metabolic breakdown of (-)-SNI-2011 was studied in vitro using rat and dog liver microsomes to assess its biotransformation. Upon oral administration, peak plasma concentrations were reached within an hour in both rats and dogs, showcasing rapid absorption and a subsequent decrease in concentration with a half-life ranging from 0.4 to 1.1 hours. Bioavailability was noted at 50% in rats and 30% in dogs. Metabolic pathways highlighted significant species differences, with both S- and N-oxidized metabolites identified in rats, but only N-oxidized metabolites in dogs. Additionally, gender differences in pharmacokinetics were observed in rats but were absent in dogs. In vitro studies pinpointed the involvement of cytochrome P450 (CYP) and flavin-containing monooxygenase (FMO) in the metabolism of (-)-SNI-2011, specifically through sulfoxidation and N-oxidation processes, respectively. CYP2D and CYP3A were identified as the primary enzymes responsible for sulfoxidation in rat liver microsomes.
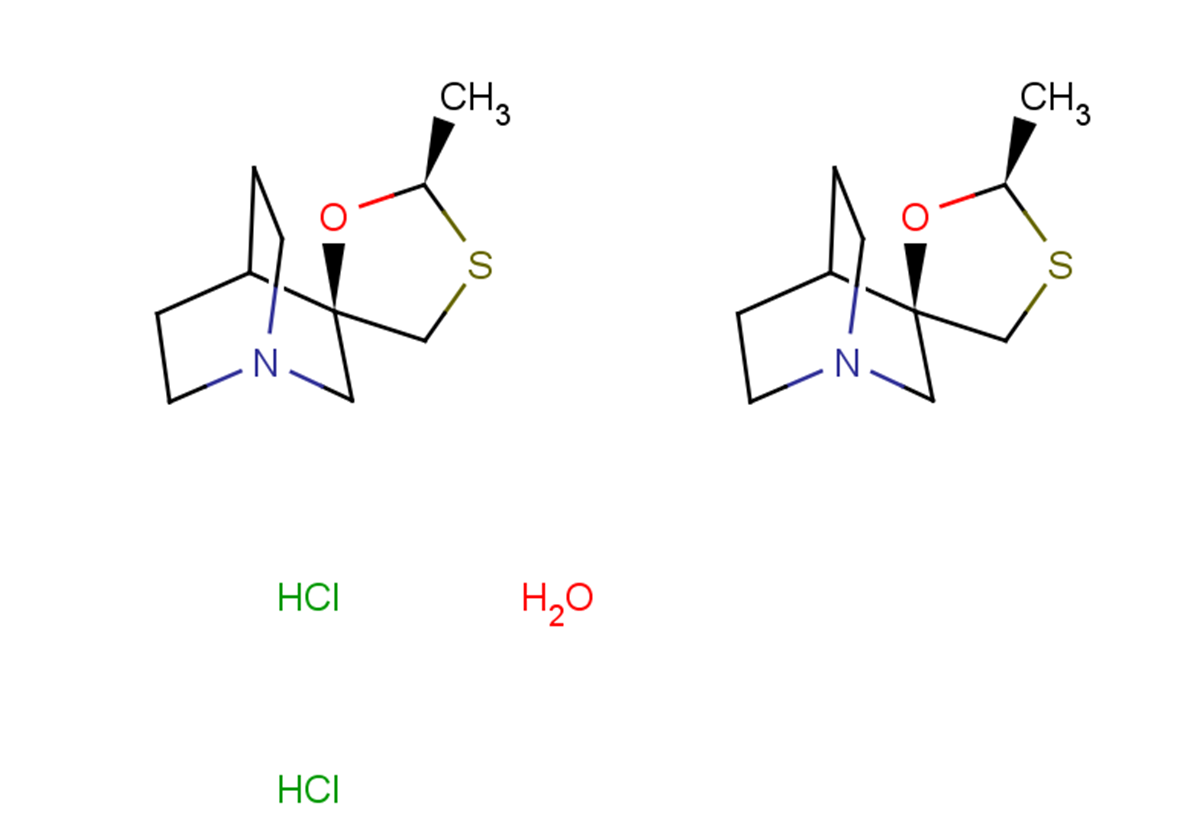
(-)-Cevimeline hydrochloride hemihydrate
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | Inquiry | 3-6 months | |
| 50 mg | Inquiry | 3-6 months | |
| 100 mg | Inquiry | 3-6 months |
Product Introduction
| Description | Cevimeline hydrochloride hemihydrate ((-)-SNI-2011), a novel muscarinic receptor agonist, is being explored as a potential treatment for xerostomia in Sjogren's syndrome, exhibiting an IC50 value indicative of its affinity for mAChR. This compound's pharmacological effects on the gastrointestinal, urinary, and reproductive systems, alongside its impact on various tissues, were thoroughly examined in species including mice, rats, guinea pigs, rabbits, and dogs. The metabolic breakdown of (-)-SNI-2011 was studied in vitro using rat and dog liver microsomes to assess its biotransformation. Upon oral administration, peak plasma concentrations were reached within an hour in both rats and dogs, showcasing rapid absorption and a subsequent decrease in concentration with a half-life ranging from 0.4 to 1.1 hours. Bioavailability was noted at 50% in rats and 30% in dogs. Metabolic pathways highlighted significant species differences, with both S- and N-oxidized metabolites identified in rats, but only N-oxidized metabolites in dogs. Additionally, gender differences in pharmacokinetics were observed in rats but were absent in dogs. In vitro studies pinpointed the involvement of cytochrome P450 (CYP) and flavin-containing monooxygenase (FMO) in the metabolism of (-)-SNI-2011, specifically through sulfoxidation and N-oxidation processes, respectively. CYP2D and CYP3A were identified as the primary enzymes responsible for sulfoxidation in rat liver microsomes. |
| Alias | (-)-SNI-2011, (-)-AF102B hydrochloride hemihydrate |
| Molecular Weight | 244.78 |
| Formula | C10H19ClNO1.5S |
| Relative Density. | 1.31g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support
Keywords

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.




