Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

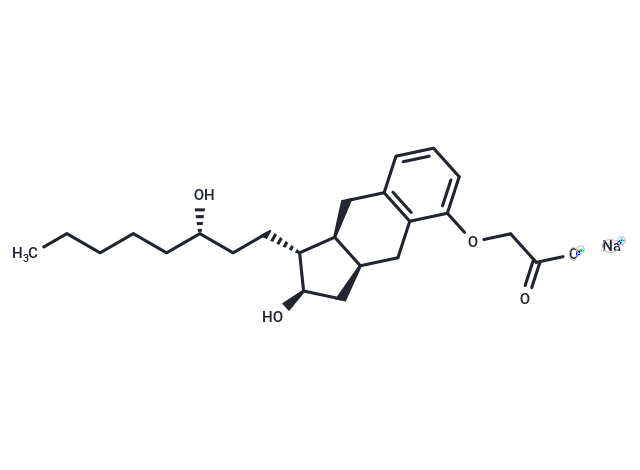
Treprostinil Sodium (UT-15) is a potent DP1, IP and EP2 agonist (EC50: 0.6/1.9/6.2 nM).
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | $47 | In Stock | |
| 5 mg | $89 | In Stock | |
| 10 mg | $143 | In Stock | |
| 25 mg | $288 | In Stock | |
| 50 mg | $466 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $98 | In Stock |
| Description | Treprostinil Sodium (UT-15) is a potent DP1, IP and EP2 agonist (EC50: 0.6/1.9/6.2 nM). |
| Targets&IC50 | RET:13 nM (Cell-free), VEGFR2:1 nM (Cell-free) |
| In vitro | In vitro enzyme experiments showed that Apatinib was an even more selective inhibitor of VEGFR-2 than sunitinib, with an IC50 of 0.001 μM and 0.005 μM, respectively. Apatinib could also potently suppress the activities of Ret, c-kit, and c-src with an IC50 of 0.013 μM, 0.429 μM, and 0.53 μM, respectively. Apatinib had no significant effects on EGFR, Her-2 or FGFR1 in concentrations up to 10 μM. Apatinib slightly inhibits proliferation of HUVEC stimulated by 20% FBS (IC50: 23.4 μM), whereas Apatinib significantly inhibits proliferation stimulated by 20 ng/mL VEGF (IC50: 0.17 μM). The IC50 values of Sunitinib are lower under the same conditions (7.4 μM and 0.034 μM, respectively). 1 μM Apatinib significantly inhibits the migration of HUVEC induced by FBS. At a concentration of 1 μM, Sunitinib also inhibits the migration of HUVEC [1]. Apatinib significantly enhanced the cytotoxicity of ABCB1 or ABCG2 substrate drugs in KBv200, MCF-7/adr, and HEK293/ABCB1 cells overexpressing ABCB1 and in S1-M1-80, MCF-7/FLV1000, and HEK293/ABCG2-R2 cells overexpressing ABCG2 (wild-type). In contrast, apatinib did not alter the cytotoxicity of specific substrates in the parental cells and cells overexpressing ABCC1. Apatinib significantly increased the intracellular accumulation of rhodamine 123 and doxorubicin in the multidrug resistance (MDR) cells. Furthermore, apatinib significantly inhibited the photoaffinity labeling of both ABCB1 and ABCG2 with [(125)I]iodoarylazidoprazosin in a concentration-dependent manner. The ATPase activity of both ABCB1 and ABCG2 was significantly increased by apatinib. However, apatinib, at a concentration that produced a reversal of MDR, did not significantly alter the ABCB1 or ABCG2 protein or mRNA expression levels or the phosphorylation of AKT and extracellular signal-regulated kinase 1/2 (ERK1/2) [2]. |
| In vivo | Once-daily oral administration of YN968D1 produced a dose-dependent inhibition of tumor growth in all tumor models examined. Statistically significant growth inhibition was obtained with 50 mg/kg?day YN968D1 in three of five tumor xenografts tested. Each tumor xenograft model was significant growth inhibited by YN968D1 at the dose of 100?kg/day. Similar tumor growth inhibition was observed (T?C%, 8% to 18%) in mice following treatment with YN968D1 at the dose of 200?kg/day [1]. There was no significant difference in tumor size between animals treated with saline, apatinib, or paclitaxel. However, the combination of apatinib and paclitaxel produced a significant inhibition of tumor growth compared with animals treated with saline, paclitaxel, or apatinib alone (P<0.05). The ratio of tumor growth inhibition by the combination was 52.7%. Furthermore, at the doses tested, no mortality or apparent decrease in body weight was observed in the combination treatment groups [2]. |
| Kinase Assay | The inhibitory activity of YN968D1 against tyrosine kinases was determined using ELISA methodology described previously. Her-2, c-kit, and c-src were activated intracellular protein tyrosine kinases expressed by Bab-to-Bac Baculovirus Expression Vector System and purified by Ni-NTA spin columns. The optical density was measured at 490 nm using VERSAmax. The inhibitory activity was expressed as IC50, which was calculated from three independent experiments by the Logit method [1]. |
| Cell Research | Immunohistochemistry was used to determine vessel density by analyzing the expression of CD31, an endothelial marker. Briefly, nude mice xenografted with NCI-H460 tumor were treated with 200 mg/kg Apatinib by the oral garage for 14 days and tumor sections were prepared from formalin-fixed and paraffin-embedded tumor tissues. Slides were treated with 3% H2O2 for 10 min and then incubated in 2% goat serum for 20 min to block the nonspecific antibody binding. Slides were stained with the anti-CD31 antibody at room temperature for 2 h, followed by treatment with biotinylated goat anti-mouse IgG and SABC complex at 37C for 30 min. Finally, diaminobenzidine tetrachloride was used for color development and the slides were counterstained with hematoxylin. Positive cells in images were measured with Image-Pro Plus software [1]. |
| Animal Research | The effects of YN968D1 on tumor growth were tested against various human tumors grown subcutaneously in BALB? cA nude mice. Tumor growth was initiated by subcutaneous inoculation of cells into mice. Tumors were allowed to establish and grow to 100–300 mm^3, at which time the mice were randomized into experimental groups. YN968D1 was administered once daily by oral gavage for the indicated periods. In combination treatment experiments, mice were administered YN968D1 alone by oral gavage; 5-FU, oxaliplatin, docetaxel and doxorubicin alone by intravenous injection; or YN968D1 in combination with each cytotoxic drug at the indicated dose and schedule. Tumor volume and body weight were monitored every other day or every 3 days, with the means indicated for groups of six (treated) or 12 (vehicle control) animals. Tumor volumes were determined by measuring the largest diameter (a) and its perpendicular (b) according to the formula (a ×b^2)? 2. The evaluation index for inhibition was the relative tumor growth ratio according to the equation: T?C (%) = mean increase of tumor volumes of treated groups?mean increase of tumor volumes of control groups ×100% [1]. |
| Alias | UT-15 |
| Molecular Weight | 412.5 |
| Formula | C23H33NaO5 |
| Cas No. | 289480-64-4 |
| Smiles | [Na+].CCCCC[C@H](O)CC[C@H]1[C@H](O)C[C@@H]2Cc3c(C[C@H]12)cccc3OCC([O-])=O |
| Relative Density. | no data available |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 25 mg/mL (60.61 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.