- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
(S)-Malic acid
(S)-Malic acid ((S)-2-Hydroxysuccinic acid) is a tart-tasting organic dicarboxylic acid found in many sour foods, such as apples, and contributes to the sourness of green apples and tartness of wine, although its concentration decreases with fruit ripeness (wikipedia). In its ionized form, it is called malate, an intermediate in the TCA cycle alongside fumarate, and can be formed from pyruvate through anaplerotic reactions. In humans, malic acid is derived from food sources and synthesized in the body via the citric acid cycle in mitochondria, playing a crucial role in energy production under both aerobic and anaerobic conditions. Under aerobic conditions, malate is oxidized to oxaloacetate, providing reducing equivalents via the malate-aspartate redox shuttle, while during anaerobic conditions, its simultaneous reduction to succinate and oxidation to oxaloacetate removes excess reducing equivalents, reversing hypoxia's inhibition of glycolysis and energy production. Studies on rats have shown that tissue malate depletes following exhaustive physical activity, suggesting that malic acid deficiency may cause physical exhaustion. Administering malic acid to rats has been shown to elevate mitochondrial malate, increasing mitochondrial respiration and energy production.
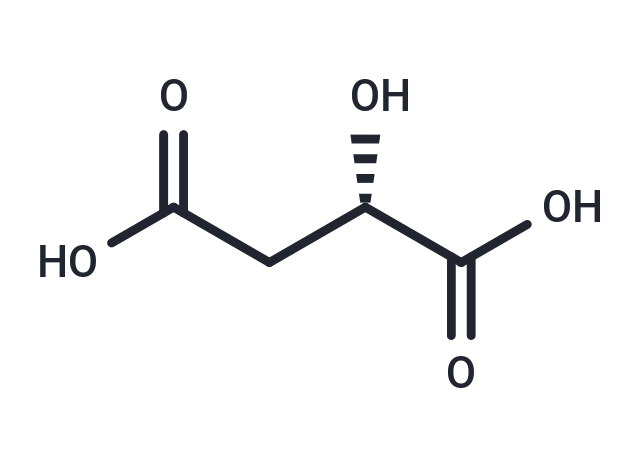
(S)-Malic acid
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 g | $29 | In Stock | |
| 10 g | $34 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock |
Product Introduction
| Description | (S)-Malic acid ((S)-2-Hydroxysuccinic acid) is a tart-tasting organic dicarboxylic acid found in many sour foods, such as apples, and contributes to the sourness of green apples and tartness of wine, although its concentration decreases with fruit ripeness (wikipedia). In its ionized form, it is called malate, an intermediate in the TCA cycle alongside fumarate, and can be formed from pyruvate through anaplerotic reactions. In humans, malic acid is derived from food sources and synthesized in the body via the citric acid cycle in mitochondria, playing a crucial role in energy production under both aerobic and anaerobic conditions. Under aerobic conditions, malate is oxidized to oxaloacetate, providing reducing equivalents via the malate-aspartate redox shuttle, while during anaerobic conditions, its simultaneous reduction to succinate and oxidation to oxaloacetate removes excess reducing equivalents, reversing hypoxia's inhibition of glycolysis and energy production. Studies on rats have shown that tissue malate depletes following exhaustive physical activity, suggesting that malic acid deficiency may cause physical exhaustion. Administering malic acid to rats has been shown to elevate mitochondrial malate, increasing mitochondrial respiration and energy production. |
| In vitro | It is shown that ME is essential for (S)-Malic acid (L-malic acid) utilization in L. casei. Moreover, deletion of either the gene encoding the histidine kinase or the response regulator of the TC system resulted in the loss of the ability to grow on (S)-Malic acid, thus indicating that the cognate TC system regulates and is essential for the expression of ME. Transcriptional analyses shows that expression of maeE is induced in the presence of (S)-Malic acid and repressed by glucose, whereas TC system expression is induced by (S)-Malic acid and is not repressed by glucose. |
| Alias | L-(-)-Malic acid, (S)-2-Hydroxysuccinic acid, (S)-(-)-HYDROXYSUCCINIC ACID |
| Molecular Weight | 134.09 |
| Formula | C4H6O5 |
| Cas No. | 97-67-6 |
| Smiles | [C@H](CC(O)=O)(C(O)=O)O |
| Relative Density. | 1.60 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 27.5 mg/mL (205.09 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
Sci Citations
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support
Keywords

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



