Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

4-Hydroxyestrone, an endogenous estrogen metabolite, can strongly protect neuronal cells against oxidative damage.
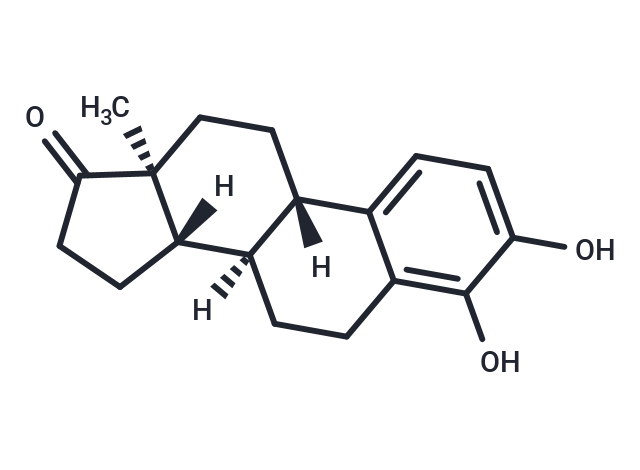
| Description | 4-Hydroxyestrone, an endogenous estrogen metabolite, can strongly protect neuronal cells against oxidative damage. |
| In vitro | Using immortalized mouse hippocampal neuronal cells as an in vitro model, 4-Hydroxyestrone, an estrone metabolite with little estrogenic activity, is found to have the strongest neuroprotective effect against oxidative neurotoxicity among 25 endogenous estrogen metabolites tested, and its protective effect is stronger than 17β-estradiol. Similarly, 4-Hydroxyestrone also exerts a stronger protective effect than 17β-estradiol against kanic acid-induced hippocampal oxidative damage in rats[1]. |
| In vivo | 4-Hydroxyestrone also exerts a stronger protective effect than 17β-estradiol against kanic acid-induced hippocampal oxidative damage in rats. Neuroprotection by 4-hydroxyestrone involves increased cytoplasmic translocation of p53 resulting from SIRT1-mediated deacetylation of p53. Analysis of brain microsomal enzymes shows that estrogen 4-hydroxylation is the main metabolic pathway in the central nervous system[1]. |
| Molecular Weight | 286.37 |
| Formula | C18H22O3 |
| Cas No. | 3131-23-5 |
| Smiles | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(O)c3CC[C@@]21[H] |
| Relative Density. | 1.241g/cm3 |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.