Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

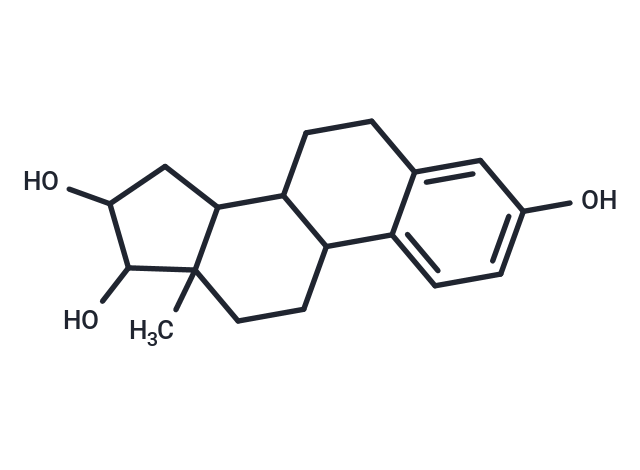
Estriol (Oestriol) is a hydroxylated metabolite of ESTRADIOL or ESTRONE that has a hydroxyl group at C3, 16-alpha, and 17-beta position. Estriol is a major urinary estrogen. During PREGNANCY, a large amount of estriol is produced by the PLACENTA.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 50 mg | $37 | In Stock | |
| 100 mg | $55 | In Stock | |
| 500 mg | $72 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock |
| Description | Estriol (Oestriol) is a hydroxylated metabolite of ESTRADIOL or ESTRONE that has a hydroxyl group at C3, 16-alpha, and 17-beta position. Estriol is a major urinary estrogen. During PREGNANCY, a large amount of estriol is produced by the PLACENTA. |
| In vitro | Intraperitoneal injection of 20 mg/kg of Estriol in rats increased the sensitivity of Kupffer cells to lipopolysaccharides (LPS) through a mechanism that depends on the elevation of portal blood endotoxin, which is caused by an increase in rat intestinal permeability, leading to a rise in CD14. When half of the rats were given an intraperitoneal pre-injection of Estriol 24 hours before administering a sub-lethal dose of LPS (5 mg/kg), 50% of these rats died within 24 hours. In mPTEN +/- mice, Estriol treatment resulted in a 187.54% increase in the relative ratio of uterine wet weight to body weight, while in wild-type mice, the same treatment led to a 176.88% increase in the ratio. |
| In vivo | Research indicates that estrogens (estrone, estradiol, and estriol) inhibit the formation of low-level Aβ oligomers associated with Alzheimer's disease, with estriol demonstrating the strongest in vitro activity. Cell-free transcription assays reveal that the anti-estrogenic activity exhibited by estriol is due to its interference with the positive cooperation binding and dimerization induced by estradiol, the binding of hER complexes with ERE, and the reduction in estradiol-dependent transcription, highlighting a dose-dependent effect. MTT assays utilizing G-1 show that in SkBr3 cells, the proliferative action induced by 100 nM G-1 is nullified in the presence of 1 μM estriol, acting as an antagonist in the GPR30-dependent pathway. |
| Cell Research | For the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cells are cultured in plastic 96-well plates under 200 μL of growth medium and at an initial density of 10,000 cells per well. Cells are washed once they have attached and further incubated in medium containing 2.5% charcoal-stripped FBS with the indicated treatment. The medium is changed every 2 days (with treatment). Where applicable, 200 ng of the indicated plasmids are transfected every 2 days before treatments using Fugene6 Reagent, as recommended by the manufacturer. Following 6 days of incubation, the assay mixture (10μL per well) containing 1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is added to each well and incubated at 37 ?C for 4 h in a 5% CO2 atmosphere. The yellow tetrazolium MTT, reduced by metabolically active cells, results in intracellular purple formazan, which is released after overnight incubation with 200 μL 1% sodium dodecyl sulfate in 0.01N HCL and quantified spectrophotometrically by reading absorbance at 570 nM using an enzyme-linked immunosorbent assay plate reader.(Only for Reference) |
| Alias | Oestriol, NSC-12169 |
| Molecular Weight | 288.38 |
| Formula | C18H24O3 |
| Cas No. | 50-27-1 |
| Smiles | CC12CCC3C(CCc4cc(O)ccc34)C1CC(O)C2O |
| Relative Density. | 1.27 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 60 mg/mL (208.06 mM), Sonication is recommended. Ethanol: 10 mg/mL (34.68 mM), Sonication is recommended. H2O: < 1 mg/mL (insoluble or slightly soluble) | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.