Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

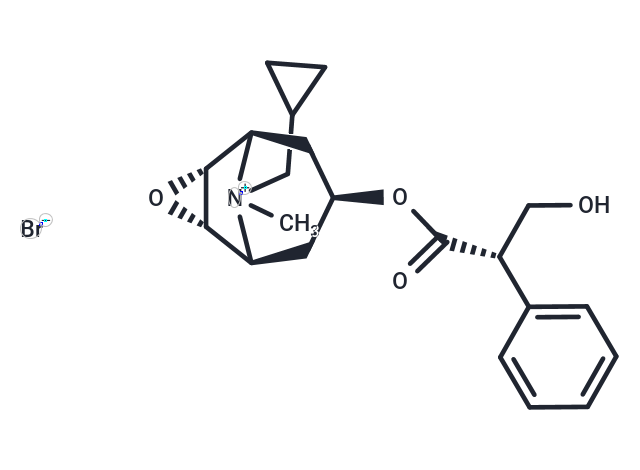
Cimetropium Bromide is a mAChR antagonist used for long-term treatment of irritable bowel syndrome.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 2 mg | $140 | 5 days | |
| 25 mg | $766 | 6-8 weeks | |
| 50 mg | $996 | 6-8 weeks | |
| 100 mg | $1,540 | 6-8 weeks |
| Description | Cimetropium Bromide is a mAChR antagonist used for long-term treatment of irritable bowel syndrome. |
| In vitro | Cimetropium reduces the release of labeled acetylcholine (ACh) elicited by electrical field stimulation, particularly when muscarinic autoinhibition is blocked, as observed in superfusion experiments using preparations preloaded with labeled choline [2]. Furthermore, it exhibits a powerful antimuscarinic action by inhibiting the contraction of longitudinal muscle preparations. As a competitive antagonist, Cimetropium Bromide effectively counteracts muscarinic-mediated contractions in isolated colonic preparations from various species, demonstrating affinity values (pA2) between 7.41 and 7.82 [1]. |
| In vivo | Cimetropium Bromide (10-100 μg/kg) counteracts colonic motor response to neostigmine administration (ID50: 27.9 μg/kg); both tonic and phasic components of contractile response are affected. Cimetropium is a potent inhibitor of large bowel motility evoked by both exogenous and endogenous stimuli when administered intravenously to conscious dogs provided with a colonic Thiry fistula. In a comparable range of doses (3-100μg/kg), the drug inhibits motor activity elicited by intraluminal distension [1]. |
| Alias | DA-3177 |
| Molecular Weight | 438.36 |
| Formula | C21H28BrNO4 |
| Cas No. | 51598-60-8 |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 50 mg/mL (114.06 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
H2O
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.