Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

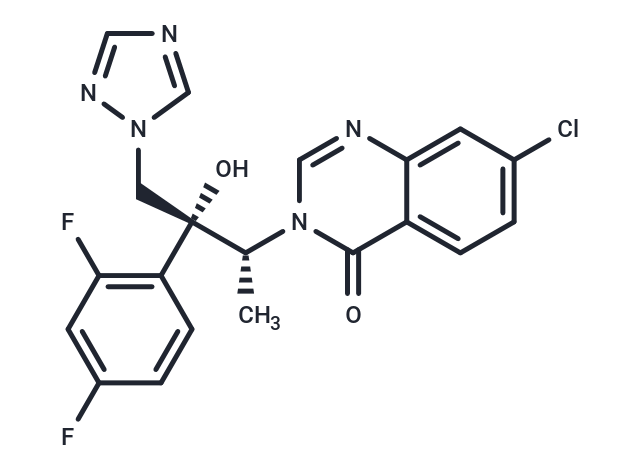
Albaconazole (W-0027) is a small molecule fungal cytochrome P450 family member 51 (fungal CYP51A1) inhibitor. It is used in the treatment of fungal infections, skin and musculoskeletal disorders, and can be used in the study of vulvovaginal candidiasis, Chagas disease and genitourinary disorders.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | $350 | In Stock | |
| 5 mg | $828 | In Stock | |
| 10 mg | $1,130 | In Stock | |
| 25 mg | $1,590 | In Stock | |
| 50 mg | $1,980 | In Stock | |
| 100 mg | $2,500 | In Stock | |
| 500 mg | $4,900 | In Stock |
| Description | Albaconazole (W-0027) is a small molecule fungal cytochrome P450 family member 51 (fungal CYP51A1) inhibitor. It is used in the treatment of fungal infections, skin and musculoskeletal disorders, and can be used in the study of vulvovaginal candidiasis, Chagas disease and genitourinary disorders. |
| In vivo | Forty participants were enrolled in this Phase I, open-label, two-sequence crossover study. Twenty participants were exposed to a single 400-mg tablet dose of albaconazole before being crossed over to a single dose of four 100-mg albaconazole capsules. The second group of 20 participants received the study products in reverse order. Blood samples were taken over 15 days post-dose to assess the plasma concentrations and pharmacokinetic parameters of albaconazole and its primary metabolite, 6-hydroxyalbaconazole. Safety was assessed throughout the study. The area under the curve (AUC) and maximum measured plasma concentration (C(max)) of the albaconazole tablet were approximately 10% and 22% lower, respectively, than for the albaconazole capsules. Statistical significance was reached for the C(max) but not for the AUC measurements (AUC(0-t) and AUC(0-inf)). Because the 90% confidence intervals based on the differences between the tablet and capsule were outside the 80%-125% range for both the C(max) and AUC, we concluded that the formulations were not bioequivalent with respect to the rate or extent of absorption. The AUC and C(max) of albaconazole after a single 400-mg oral dose administered as a tablet formulation were lower than those of a capsule formulation. Albaconazole tablets and capsules cannot, therefore, be considered bioequivalent.[1] |
| Alias | W-0027, W 0027, UR-9825, UR9825 |
| Molecular Weight | 431.82 |
| Formula | C20H16ClF2N5O2 |
| Cas No. | 187949-02-6 |
| Smiles | [C@@]([C@@H](C)N1C(=O)C=2C(N=C1)=CC(Cl)=CC2)(CN3C=NC=N3)(O)C4=C(F)C=C(F)C=C4 |
| Relative Density. | 1.48g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 55 mg/mL (127.37 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.