Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Doxorubicin (Adriamycin) is a Topoisomerase II (Top2) inhibitor with antineoplastic activity.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 10 mg | Inquiry | Backorder |
| Description | Doxorubicin (Adriamycin) is a Topoisomerase II (Top2) inhibitor with antineoplastic activity. |
| Targets&IC50 | Topo I:0.8 μM (IC50), Topo II:2.67 μM (IC50) |
| In vitro | The combination of Doxorubicin and Simvastatin at the highest tested concentrations (2 μM and 10 μM, respec-tively) kills 97% of the Hela cells[2]. |
| In vivo | In an experiment, mice with PC3 xenografts received injections of Doxorubicin at dosages of 2, 4, or 8 mg/kg, and tumor volume was monitored. The 2 mg/kg dose did not inhibit tumor growth, but doses of 4 mg/kg and 8 mg/kg initially delayed growth, significantly reducing c-FLIP levels in the tumors (p<0.05 on days 18 and 22)[3]. In a separate study with rats, treatments involved a single intraperitoneal injection of 10 mg/kg Doxorubicin, ten daily injections of 1 mg/kg, or five weekly injections of 2 mg/kg, resulting in an 80% mortality rate by day 28 for the first group, and on days 107 and 98 for the latter groups, respectively. Furthermore, fractional shortening—a measure of heart function—decreased by 30% in the first group at week 2, 55% in the second group at week 13, and 42% in the third group at week 13[4]. |
| Cell Research | Doxorubicin is dissolved in stock solutions (1 mM) and serially diluted with RPMI 1640 media (0.1, 1, and 2 μM)[2]. 160 μL of Hela cells suspension (3×104 cell/mL) is dispensed into three 96-well U-bottom microplates and incubated for 24 h at 37°C in a fully humidified atmosphere of 5% CO2. In plate 1, serial dilutions of Doxorubicin (20 μL; final concentration, 0.1-2 μM) and Simvastatin (20 μL; final concentration, 0.25-2 μM) are added to a final volume of 200 μL and incubated for another 72 h. In plates 2 and 3 serial dilutions of each drug (Simvastatin or Doxorubicin, 40 μL) are added. After an incubation period of 24 h, the medium is aspirated and the cells are washed in PBS. Then, serial dilutions of other drug (40 μL) are added and supplemented with culture medium to a final volume of 200 μL, and incubated for 48 h. Doxorubicin and Simvastatin are used individually as positive controls (40 μL in each well), and the cells treated only with solvent are considered as negative controls. To evaluate cell survival, 20 μL of MTT solution (5 mg/mL in PBS) is added to each well and incubated for 3 h. Then the media is replaced with 150 μL of DMSO, and complete solubilization of formazan crystals is achieved by repeated pipetting of the solution. Absorbance is then determined at 540 nm by an ELISA plate reader. Each drug concentration is assayed in 4 or 8 wells and repeated 3 times. The cytotoxic/cytostatic effect of Doxorubicin is expressed as the relative viability (% control) and calculated. Percentage of cell survival in the negative control is assumed as 100. Relative viability=(experimental absorbance-background absorbance)/ (absorbance of untreated controls-background absorbance)×100 %[2]. |
| Alias | Hydroxydaunorubicin, DOX, Adriamycin |
| Molecular Weight | 543.52 |
| Formula | C27H29NO11 |
| Cas No. | 23214-92-8 |
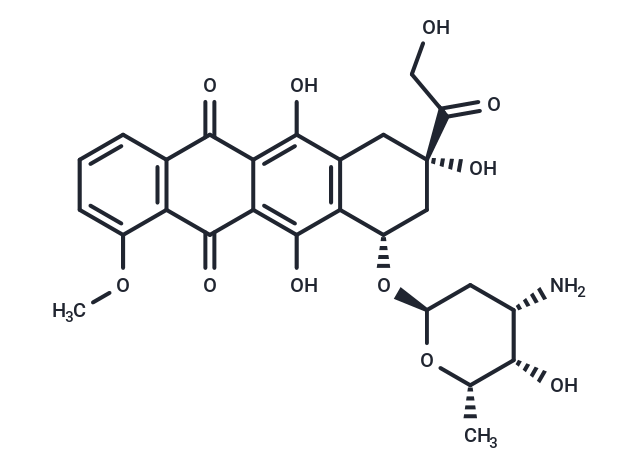
| Smiles | COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |
| Relative Density. | 1.61 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 50 mg/mL (91.99 mM), Sonication is recommended. DMSO: 10 mM, Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO/H2O
H2O
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.