Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

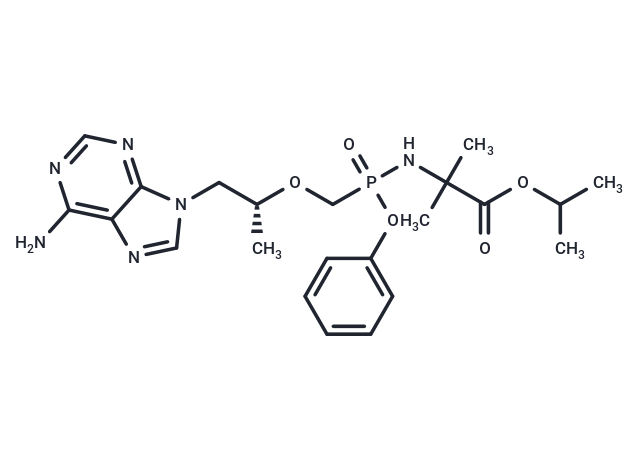
Tenofovir amibufenamide (HS-10234) is a Tenofovir prodrug, an antiviral compound with oral activity. Tenofovir amibufenamide inhibits hepatitis B virus (HBV) and can be used in chronic hepatitis B (CHB) studies.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | $233 | In Stock | |
| 5 mg | $579 | In Stock | |
| 10 mg | $868 | In Stock | |
| 25 mg | $1,390 | In Stock | |
| 50 mg | $1,930 | In Stock | |
| 100 mg | $2,650 | In Stock |
| Description | Tenofovir amibufenamide (HS-10234) is a Tenofovir prodrug, an antiviral compound with oral activity. Tenofovir amibufenamide inhibits hepatitis B virus (HBV) and can be used in chronic hepatitis B (CHB) studies. |
| Targets&IC50 | Anti-HBV:7.29 ± 0.71 nM(EC50) |
| In vitro | TMF and Tenofovir amibufenamide exhibited significantly stronger inhibition of HBV DNA replication than did TDF in HBV-positive HepG2.2.15 cells. The anti-HBV activity of TMF was slightly stronger than Tenofovir amibufenamide after 9 days of treatment (EC50 7.29 ± 0.71 nM vs. 12.17 ± 0.56 nM). The callback effects of the three TFV ester prodrugs were ranked as TMF > Tenofovir amibufenamide > TDF. These advantages of TMF were believed to be attributed to its greater bioavailability in preclinical animals (SD rats, C57BL/6 mice and beagle dogs) and better target loading, especially in terms of the higher hepatic level of the pharmacologically active metabolite TFV-DP, which was tightly related to anti-HBV efficacy. [1] |
| In vivo | Safety was evaluated thoroughly focusing on bone, renal, and metabolic parameters between Tenofovir amibufenamide (25 mg, for 96 weeks) and TDF group. Non-indexed estimated glomerular filtration rate for renal safety assessment was adopted, while a smaller decline of which was seen in the Tenofovir amibufenamide group than in the TDF group (p=0.01). For bone mineral density, patients receiving Tenofovir amibufenamide displayed significantly lower reduction levels in the densities of spine, hip, and femur neck at week 96 than those receiving TDF. In addition, the lipid parameters were stable after week 48 in all groups while weight change still showed the opposite trend. Tenofovir amibufenamide maintained similar efficacy at week 96 compared with TDF with continued superior bone and renal safety profiles.[2] |
| Alias | HS-10234 |
| Molecular Weight | 490.49 |
| Formula | C22H31N6O5P |
| Cas No. | 1571076-26-0 |
| Smiles | C([C@H](OCP(OC1=CC=CC=C1)(NC(C(OC(C)C)=O)(C)C)=O)C)N2C=3C(N=C2)=C(N)N=CN3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 180.0 mg/mL (367.0 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.