Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

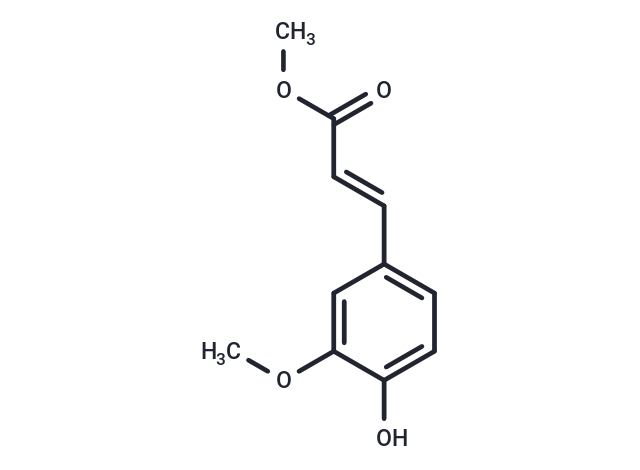
FERULIC ACID METHYL ESTER (Methyl ferulate) is a hydroxycinnamic acid that is abundant in plants and originally derived from giant fennel (F. communis). This naturally-occurring phenolic has antioxidant activities that provide protection against inflammation and cancer. Ferulic acid methyl ester is a lipophilic derivative of ferulic acid, demonstrating increased ability to cross cell membranes.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 mg | 27 € | In Stock | |
| 1 mL x 10 mM (in DMSO) | 27 € | In Stock |
| Description | FERULIC ACID METHYL ESTER (Methyl ferulate) is a hydroxycinnamic acid that is abundant in plants and originally derived from giant fennel (F. communis). This naturally-occurring phenolic has antioxidant activities that provide protection against inflammation and cancer. Ferulic acid methyl ester is a lipophilic derivative of ferulic acid, demonstrating increased ability to cross cell membranes. |
| In vitro | Ferulic acid methyl ester has less antioxidant capacity than ferulic acid in neuronal PC12 cells (IC50 = 74.7 μM for ferulic acid ethyl ester vs. 44.6 μM for ferulic acid, 2,2-diphenyl-1-picrylhydrazyl radical scavenging). Ferulic acid methyl ester, at 10-25 μg/ml, inhibits the release of pro-inflammatory cytokines, blocks the expression of COX-2, and reduces nitric oxide generation from LPS-stimulated macrophages. |
| Kinase Assay | DPPH radical scavenging activity of caffeic acid and ferulic acid derivatives was assessed as previously described withsome modifications. Briefly, the test compound was dissolved in dimethyl sulfoxide and 4 different concentrations weremixed with a methanolic solution of DPPH 100 mM in duplicate. After 30 min of incubation at room temperature in the dark, theabsorbance at 517 nm was measured by a spectrophotometer . The concentrations (in the range 1-100 μM) were carefully chosen for each compound in order to produce a suitable doseeresponse curve. The percent inhibition of the radical was calculated based on the absorbance of the mixture compared to the absorbance of DPPH solution alone. |
| Cell Research | Hydrogen peroxide (8.8 M solution) stored at 4 C was firstdiluted in PBS to prepare a 100 mM solution on the day of theexperiment. This was further diluted in growth medium to preparethe final working solution. PC12 cells were plated in collagencoated96-well microplates at a density of 5 105 cells/ml(100 μl per well). Blank wells contained only growth medium forbackground correction. After 48 h of incubation to allow for cellattachment, 20 μl of growth medium supplemented with differentconcentrations of HCAs were added in triplicate wells and preincubatedfor 1 h. Maximum concentration of DMSO in the wellswas kept below 0.2%. Afterwards, 20 μl of H2O2 solution was added.The concentration of H2O2 in the well was 75 μM. After anotherhour, the medium was replaced with fresh one and cells wereincubated overnight. In the end, the medium was replaced with30 μl of MTT 0.5 mg/ml dissolved in RPMI without phenol red.Formazan crystals were solubilised in 200 μl DMSO after 1.5 h ofincubation at 37 C. |
| Alias | Methyl ferulate, Methyl 4'-hydroxy-3'-methoxycinnamate |
| Molecular Weight | 208.21 |
| Formula | C11H12O4 |
| Cas No. | 2309-07-1 |
| Smiles | COC(=O)\C=C\c1ccc(O)c(OC)c1 |
| Relative Density. | 1.204 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (240.14 mM), Sonication is recommended. Ethanol: 20 mg/mL (96.06 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.