Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

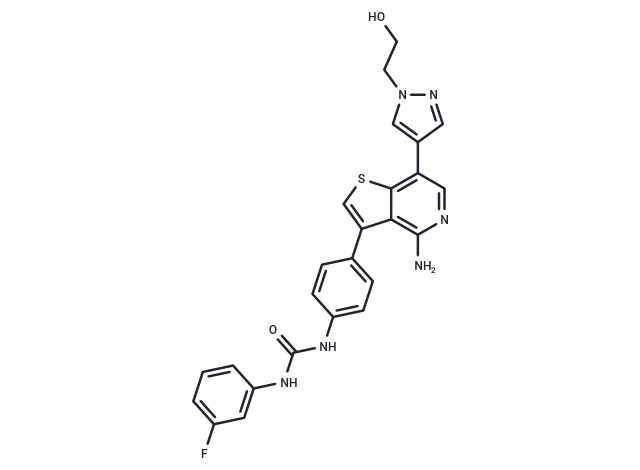
Ilorasertib (ABT-348) (ABT-348) is an ATP-competitive multitargeted kinase inhibitor, which inhibits Aurora A/Aurora B/Aurora C (IC50s: 120 nM/7 nM/1 nM). It also suppresses RET tyrosine kinase, PDGFRβ, and Flt1 (IC50s: 7 nM, 3 nM, and 32 nM).
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | $33 | In Stock | |
| 5 mg | $75 | In Stock | |
| 10 mg | $110 | In Stock | |
| 25 mg | $178 | In Stock | |
| 50 mg | $293 | In Stock | |
| 100 mg | $429 | In Stock | |
| 200 mg | $597 | In Stock |
| Description | Ilorasertib (ABT-348) (ABT-348) is an ATP-competitive multitargeted kinase inhibitor, which inhibits Aurora A/Aurora B/Aurora C (IC50s: 120 nM/7 nM/1 nM). It also suppresses RET tyrosine kinase, PDGFRβ, and Flt1 (IC50s: 7 nM, 3 nM, and 32 nM). |
| Targets&IC50 | PDGFRβ:13 nM, Aurora C:1 nM, Aurora A:120 nM, Aurora B:7 nM, PDGFRα:11 nM, VEGFR1:1 nM, FLT3:1 nM, VEGFR2:2 nM |
| In vitro | In addition to targeting Aurora kinases, Ilorasertib is a potent inhibitor of the VEGFR and PDGFR kinase families and, to a lesser extent, the Src family of cytoplasmic tyrosine kinases. Ilorasertib induces a concentration-dependent increase in the extent and number of two NSCLC cell lines exhibiting polyploidy (EC50: 5, 10 nM). Ilorasertib shows antiproliferative activity against BCR-ABL expressing CML cells and cells expressing the Gleevec-resistant BCR-ABL T315I mutation (IC50: 47, 260 nM) [2]. |
| In vivo | Ilorasertib inhibits the VEGF response with a potency (ED50: 0.2 mg/kg, i.v.) in a uterine edema model. Ilorasertib (25 mg/kg, s.c.) leads to an inhibition of histone H3 phosphorylation in circulating tumor cells obtained from an engrafted leukemia model. Ilorasertib (20 mg/kg, p.o.) inhibits the growth of established tumors and causes regression of advanced tumors in human xenograft models [2]. Ilorasertib demonstrates significant antitumor efficacy in both solid and hematological xenograft models after intravenous, mini-pump or parenteral once-weekly dosing [3]. |
| Cell Research | Noncycling primary HUVEC are used to assess the effect of Ilorasertib on nonproliferating cells. Cells (35,000/well) are seeded in growth medium in a 96-well tissue culture plate, and after 2 days, the medium is changed and the cells are treated with Ilorasertib. After an additional 3 days, cell viability is measured with Cell TiterGlo reagent [2]. |
| Animal Research | For flank xenograft models, cells are suspended in PBS, mixed with Matrigel (phenol red-free) in a ratio of 1:4 (v/v), and inoculated into the flank of female SCID/beige mice (5 million cells per site). Inoculated mice are randomized into groups of 10, and treatment is initiated when mean tumor volume is approximately 0.4 cm^3 or 0.5 cm^3. Tumor growth in the flank is assessed by measuring tumor size with calipers and calculating volume using the formula (L × W2/2). Study groups are terminated before tumor volume reaches 3 cm^3. Inhibition of tumor growth is assessed at the time the vehicle-treated group is terminated by calculating the ratio of the mean volume of the test drug group to the mean volume of the untreated (control) group (T/C) and calculating the percentage of inhibition of control [(1 ? T/C) × 100]. The dosing formulation of test agents is prepared by stepwise addition, with mixing, of the following reagents: EtOH, Tween 80, polyethylene glycol 400, and 2% hydroxypropyl methylcellulose (2:5:20:73, v/v). The dosing volume is 10 mL/kg [2]. |
| Alias | ABT-348 |
| Molecular Weight | 488.54 |
| Formula | C25H21FN6O2S |
| Cas No. | 1227939-82-3 |
| Smiles | NC1=C2C(=C(C=N1)C3=CN(CCO)N=C3)SC=C2C4=CC=C(NC(NC5=CC(F)=CC=C5)=O)C=C4 |
| Relative Density. | 1.47 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 55 mg/mL (112.58 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.