Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Paromomycin binds specifically to the RNA oligonucleotide at the A site of bacterial 30S ribosomes, thereby causing misreading and premature termination of translation of mRNA and inhibition of protein synthesis followed by cell death. Paromomycin Sulfate (Aminosidine sulfate) is the sulfate salt form of paromomycin, a structural derivative of neomycin, an aminoglycoside antibiotic with amebicidal and bactericidal effects against predominantly aerobic gram-negative bacteria.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 500 mg | $41 | In Stock |
| Description | Paromomycin binds specifically to the RNA oligonucleotide at the A site of bacterial 30S ribosomes, thereby causing misreading and premature termination of translation of mRNA and inhibition of protein synthesis followed by cell death. Paromomycin Sulfate (Aminosidine sulfate) is the sulfate salt form of paromomycin, a structural derivative of neomycin, an aminoglycoside antibiotic with amebicidal and bactericidal effects against predominantly aerobic gram-negative bacteria. |
| In vitro | In both clinical cases and experimental models of cutaneous leishmaniasis (CL), lesions caused by L. major show a faster and more complete recovery when treated with paromomycin ointment as compared to those caused by L. panamensis and L. amazonensis. |
| In vivo | Paromomycin, an aminoglycoside antibiotic, exhibits robust antimicrobial activity against a broad spectrum of Gram-positive bacteria, Gram-negative bacteria, some protozoa, and tapeworms. In vitro analysis of amastigote sensitivity within a mouse macrophage model indicated that L. tropica and the L. major strains (ED50s: 1~5 μM) are more sensitive to Paromomycin than L. mexicana (ED50: 39 μM) and L. braziliensis (ED50: <12 μM). The L. donovani strain demonstrates moderate sensitivity (ED50: 6~18 μM), with the exception of the Indian strain, DD8, exhibiting significantly reduced susceptibility (ED50 >150 μM). |
| Kinase Assay | Concentration–response and kinetic studies: The microsomal protein (30 μg), [1β-3H]androstenedione (6.6 × 105 dpm) and NADPH (270 μM) are used for the concentration–response experiment with an incubation time of 20 minutes. The Aminoglutethimide is initially tested at 10 μM and 100 μM concentrations, followed by a full concentration–response study with at least 8 concentrations ranging from 0.01 μM to 160 μM. For the initial velocity study the concentration of [1β-3H]androstenedione is varied from 7.5 to 100 nM and the incubation time is set to 5 minutes. The tritiated water formed during the conversion of the tritiated substrate, [1β-3H]androstenedione, to estrone is quantified by liquid scintillation counting. Each assay is performed three times in duplicate and the results are treated by nonlinear regression analysis allowing the determination of the half-maximal inhibitory concentration (IC50). |
| Alias | Paromomycin sulfate salt, Aminosidine sulfate |
| Molecular Weight | 713.71 |
| Formula | C23H47N5O18S |
| Cas No. | 1263-89-4 |
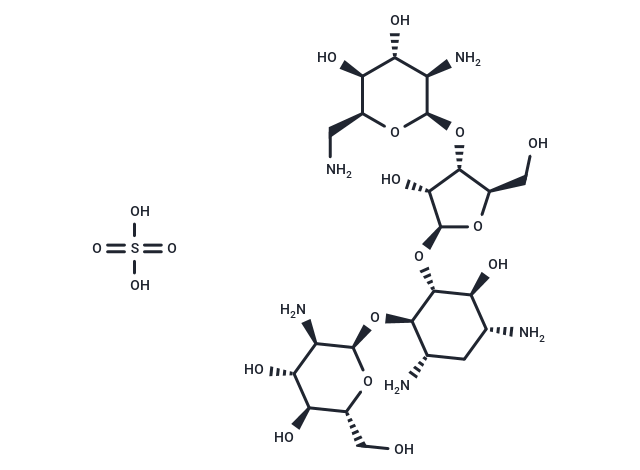
| Smiles | OS(O)(=O)=O.NC[C@@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@@H](O[C@@H]3[C@@H](O)[C@H](N)C[C@H](N)[C@H]3O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3N)[C@@H]2O)[C@H](N)[C@@H](O)[C@@H]1O |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | H2O: 10 mM, Sonication is recommended. DMSO: Insoluble | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
H2O
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.