Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Vanillyl Alcohol (3-Methoxy-4-hydroxybenzyl alcohol) possesses anti-angiogenic, anticonvulsive, anti-inflammatory, anti-oxidant, neuroprotective, and anti-nociceptive activities. Vanillyl alcohol can effectively inhibit the cytotoxicity and improved cell viability in 1-methyl-4-phenylpyridinium (MPP+)-induced MN9D dopaminergic cells, it also can attenuate the elevation of reactive oxygen species (ROS) levels, decrease in the Bax/Bcl-2 ratio and poly (ADP-ribose) polymerase proteolysis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 mg | $42 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $46 | In Stock |
| Description | Vanillyl Alcohol (3-Methoxy-4-hydroxybenzyl alcohol) possesses anti-angiogenic, anticonvulsive, anti-inflammatory, anti-oxidant, neuroprotective, and anti-nociceptive activities. Vanillyl alcohol can effectively inhibit the cytotoxicity and improved cell viability in 1-methyl-4-phenylpyridinium (MPP+)-induced MN9D dopaminergic cells, it also can attenuate the elevation of reactive oxygen species (ROS) levels, decrease in the Bax/Bcl-2 ratio and poly (ADP-ribose) polymerase proteolysis. |
| Kinase Assay | For experiments with FAAH, rat liver homogenates, mouse brain homogenates and membranes from COS7 cells transfected with the human enzyme are used. Frozen (?80°C) livers from adult C57BL/6 mice and frozen brains (minus cerebella) from adult Wistar or Sprague-Dawley rats are thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7. The homogenates are centrifuged at ~35000×g for 20 min at 4°C. After resuspension in buffer followed by recentrifugation and a second resuspension in buffer, the pellets are incubated at 37°C for 15 min. This incubation is undertaken in order to hydrolyse all endogenous FAAH substrates. The homogenates are then centrifuged as above, recentrifuged and resuspended in 50 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA and 3 mM MgCl2. The homogenates are then frozen at ?80°C in aliquots until used for assay. FAAH is assayed in the homogenates and in the COS7 cell membranes using 0.5 μM (unless otherwise stated) [3H]AEA labelled in the ethanolamine part of the molecule. Blank values are obtained by the use of buffer rather than homogenate. In the experiments comparing effects of Biochanin A upon FAAH and FAAH-2, the same assay is used but with 16 nM [3H]oleoylethanolamide ([3H]OEA) as substrate and with an incubation phase at room temperature. The choice of OEA rather than AEA for FAAH-2 is motivated by the relative rates of hydrolysis: OEA is metabolized four times faster than AEA by FAAH-2, whereas for FAAH the rate of hydrolysis of OEA is about a third of that for AEA. When 0.5 μM [3H]AEA is used as substrate, assay conditions for rat brain and mouse liver are chosen so that <10% of added substrate is metabolized. For the human FAAH samples, <5% of the [3H]AEA is metabolized in all cases. For 16 nM [3H]OEA, a limited supply of an expensive ligand meant that optimization is not possible, and the amount of substrate utilized is higher (34±1 and 0.5±0.1% for FAAH and its corresponding mock-transfected, respectively; 40±2 and 21±0.4 for FAAH-2 and its corresponding mock-transfected respectively)[1]. |
| Alias | Vanillin alcohol, Vanillic alcohol, 4-Hydroxy-3-methoxybenzyl alcohol, 4-Hydroxy-3-methoxybenzenemethanol, 3-Methoxy-4-hydroxybenzyl alcohol |
| Molecular Weight | 154.16 |
| Formula | C8H10O3 |
| Cas No. | 498-00-0 |
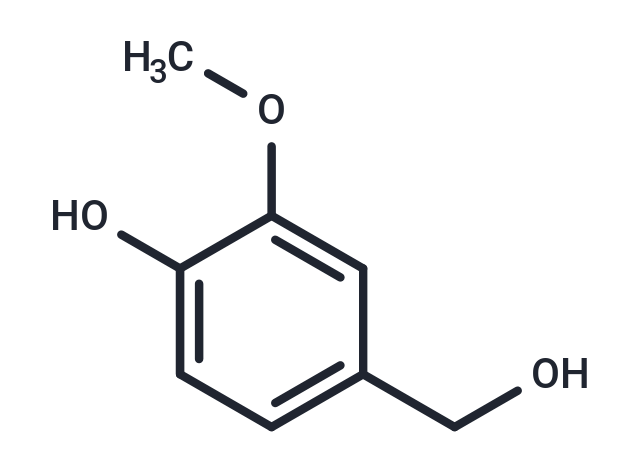
| Smiles | O(C)C1=CC(CO)=CC=C1O |
| Relative Density. | 1.226 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 45 mg/mL (291.9 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.