Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

2-Methoxyestradiol (2-ME2) is an orally bioavailable estradiol metabolite with potential antineoplastic activity. 2-Methoxyestradiol inhibits angiogenesis by reducing endothelial cell proliferation and inducing endothelial cell apoptosis. This agent also inhibits tumor cell growth by binding to tubulin, resulting in antimitotic activity, and by inducing caspase activation, resulting in cell cycle arrest in the G2 phase, DNA fragmentation, and apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $37 | In Stock | |
| 50 mg | $50 | In Stock | |
| 100 mg | $58 | In Stock | |
| 200 mg | $82 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $30 | In Stock |
| Description | 2-Methoxyestradiol (2-ME2) is an orally bioavailable estradiol metabolite with potential antineoplastic activity. 2-Methoxyestradiol inhibits angiogenesis by reducing endothelial cell proliferation and inducing endothelial cell apoptosis. This agent also inhibits tumor cell growth by binding to tubulin, resulting in antimitotic activity, and by inducing caspase activation, resulting in cell cycle arrest in the G2 phase, DNA fragmentation, and apoptosis. |
| In vitro | 2-Methoxyestradiol (60 mg/kg/day) can reduce tumor size by fourfold, and increasing the dose tenfold further reduces it by 23 times. This compound demonstrates dose-dependent therapeutic efficacy in the 9L rat glioma model (9L-V6R), significantly decreasing HIF-1 activity and inhibiting tumor growth. |
| In vivo | 2-Methoxyestradiol (0.5 μM) inhibits the TGF-β3-induced expression of Type I collagen (Col I) (αI), Col III (αI), connective tissue growth factor, plasminogen activator inhibitor, and α-smooth muscle actin. In MDA-MB-231 cells, it suppresses the transcriptional activation of HIF-1-regulated target genes without affecting HIF-1α transcription itself. It inhibits cell proliferation in breast cancer cell line MDA-MB-435 (IC50: 1.38 μM) and ovarian cancer cell line SK-OV-3 (IC50: 1.79 μM). Furthermore, in rat aortic smooth muscle A-10 cells, 2-Methoxyestradiol prevents microtubule disassembly (EC50: 7.5 μM) and mitigates TGF-β3-induced Smad2/3 phosphorylation and nuclear translocation, as well as inhibiting the activation of the TGF-β3-induced PI3K/Akt/mTOR pathway. Additionally, it suppresses cell proliferation in MCF-7 (IC50: 52 μM) and BM (IC50: 8 μM) cells. |
| Kinase Assay | Microtubule depolymerizing activity: The effects of 2-Methoxyestradiol on cellular microtubule depolymerization are determined by indirect immunofluorescence techniques in rat aortic smooth muscle A-10 cells. Microtubules are visualized using a β-tubulin antibody. Three viewers determines the percent microtubule loss for each treatment concentration. The data are averaged and plotted as percent microtubule loss versus drug concentration and the EC50s for microtubule depolymerization calculated from the log dose–response curves. |
| Cell Research | The sulforhodamine B (SRB) assay is used to evaluate the antiproliferative activity of 2-Methoxyestradiol in the MDA-MB-435 and SK-OV-3 cell lines. Cells a plated into 96-well plates and allowed to grow and attach for 24 hours followed by addition of 2-Methoxyestradiol or vehicle controls. The cells are incubated with drugs for 48 hours and then the cellular protein is fixed, stained, and concentration determined by absorbance at 560 nm. Log dose–response curves are constructed for each experiment and the IC50 for inhibition of proliferation determined.(Only for Reference) |
| Alias | NSC-659853, 2-MeOE2, 2-ME2 |
| Molecular Weight | 302.41 |
| Formula | C19H26O3 |
| Cas No. | 362-07-2 |
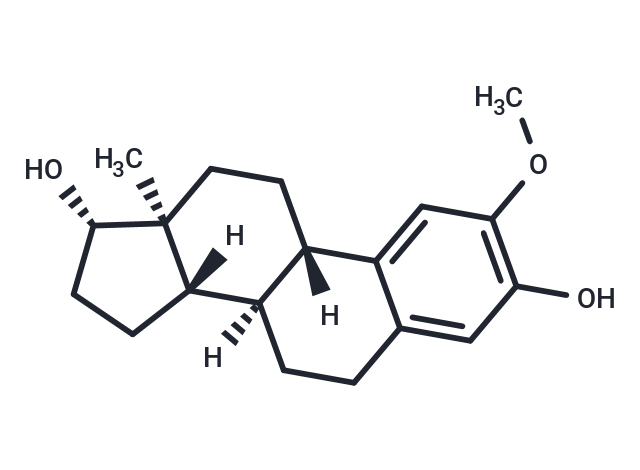
| Smiles | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(OC)c(O)cc3CC[C@@]21[H] |
| Relative Density. | 1.178 g/cm3 (Predicted) |
| Storage | store under nitrogen | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 45 mg/mL (148.8 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.