Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Dorsomorphin dihydrochloride (BML-275 2HCl) is a potent, selective and ATP-competitive AMPK inhibitor (Ki: 109 nM) and does not exhibit significant activity on structurally related kinases.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 2 mg | $30 | In Stock | |
| 5 mg | $47 | In Stock | |
| 10 mg | $64 | In Stock | |
| 25 mg | $137 | In Stock | |
| 50 mg | $247 | In Stock | |
| 100 mg | $446 | In Stock | |
| 500 mg | $987 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $52 | In Stock |
| Description | Dorsomorphin dihydrochloride (BML-275 2HCl) is a potent, selective and ATP-competitive AMPK inhibitor (Ki: 109 nM) and does not exhibit significant activity on structurally related kinases. |
| Targets&IC50 | AMPK:109 nM (cell free) |
| In vitro | Dorsomorphin (compound C) is a potent reversible inhibitor that is competitive with ATP, with Ki = 109 ± 16 nM in the absence of AMP. Incubation of cultured hepatocytes with compound C inhibited ACC inactivation by either AICAR or metformin [1]. Compound C suppressed 2-deoxy-D-glucose (2DG)-induced GRP78 promoter activity in a dose-dependent manner but had little effect on tunicamycin-induced GRP78 promoter activity. Compound C also suppressed GRP78 promoter activity induced by glucose withdrawal [2]. |
| In vivo | 6 h after dorsomorphin was administered intravenously, hepatic hepcidin mRNA levels were reduced to one-third of that of vehicle-injected mice. Alterations in hepcidin levels affect serum iron concentrations within 24 h via the altered mobilization of intracellular iron by ferroportin33. Administration of dorsomorphin over 24 h led to a 60% increase in total serum iron concentrations [3]. |
| Kinase Assay | Liver AMPK was partially purified from male SD rats to the blue-Sepharose step. The 100-μl reaction mixture contained 100 μM AMP, 100 μM ATP (0.5 μCi 33P-ATP per reaction), and 50 μM SAMS in a buffer (40 mM HEPES, pH 7.0, 80 mM NaCl, 0.8 mM EDTA, 5 mM MgCl2, 0.025% BSA, and 0.8 mM DTT). The reaction was initiated with the addition of the enzyme. After a 30-minute incubation at 30°C, the reaction was stopped by addition of 80 μl 1% H3PO4. Aliquots (100 μl) were transferred to 96-well MultiScreen plates. The plate was washed three times with 1% H3PO4 followed by detection in a Top-count. The in vitro AMPK inhibition data obtained with compound C — (6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyyrazolo[1,5-a] pyrimidine — were fit to the following equation for competitive inhibition by nonlinear regression using a least-squares Marquardt algorithm in a computer program written by N. Thornberry of Merck Research Laboratories: Vi/Vo = (Km + S)/[S + Km × (1 + I/Ki)], where Vi is the inhibited velocity, Vo is the initial velocity, S is the substrate (ATP) concentration, Km is the Michaelis constant for ATP, I is the inhibitor (compound C) concentration, and Ki is the dissociation constant for compound C [1]. |
| Cell Research | C2C12 cells were seeded into 96-well plates at 2,000 cells per well in DMEM supplemented with 2% FBS. Wells were treated in quadruplicate with BMP ligands and dorsomorphin or vehicle. Cells were harvested after 5 d in culture with 50 μl Tris-buffered saline, 1% Triton X-100. Lysates were added to p-nitro-phenylphosphate reagent in 96-well plates for 1 h, and alkaline phosphatase activity expressed as absorbance at 405 nM. Cell viability and quantity were measured by Cell-titer Glo and binding of nuclear dye CyQuant, respectively, using replicate wells treated identically to those used for alkaline phosphatase measurements [3]. |
| Animal Research | 12-week-old C57BL/6 mice raised on a standard diet were injected via the tail vein with 0.2 g kg?1 of dextran (average MW = 5,000) or 0.2 g kg?1 of iron-dextran USP. Dextran was injected with vehicle only, whereas iron-dextran was injected with either vehicle or dorsomorphin (10 mg/kg). 1 h after injection, mice were killed and liver segments were collected in 500 μl of SDS-lysis buffer and mechanically homogenized. 20 μl of liver extracts were resolved by SDS-PAGE and immunoblotted. Total RNA was harvested using Trizol from mechanically homogenized mouse livers (6 h after injection with a single intraperitoneal dose of dorsomorphin (10 mg/kg) or DMSO) [3]. |
| Alias | Dorsomorphin (Compound C) 2HCl, Compound C dihydrochloride, Compound C 2HCl, BML-275 dihydrochloride, BML-275 2HCl |
| Molecular Weight | 472.41 |
| Formula | C24H25N5O·2HCl |
| Cas No. | 1219168-18-9 |
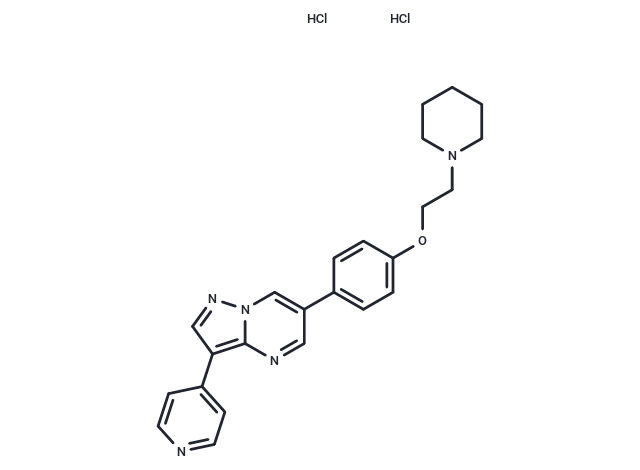
| Smiles | Cl.Cl.C(CN1CCCCC1)Oc1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccncc1 |
| Relative Density. | no data available |
| Storage | store under nitrogen | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 6.88 mg/mL (14.55 mM), Sonication is recommended. H2O: 47.2 mg/mL (99.91 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO/H2O
H2O
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.