Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Glecaprevir (ABT-493) is an HCV NS3/4A protease inhibitor, (IC50s: 3.5-11.3 nM).
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 2 mg | $32 | In Stock | |
| 5 mg | $52 | In Stock | |
| 10 mg | $77 | In Stock | |
| 25 mg | $158 | In Stock | |
| 50 mg | $288 | In Stock | |
| 100 mg | $448 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $73 | In Stock |
| Description | Glecaprevir (ABT-493) is an HCV NS3/4A protease inhibitor, (IC50s: 3.5-11.3 nM). |
| Targets&IC50 | HCV NS3/4A protease:3.5-11.3 nM |
| In vitro | Glecaprevir (ABT-493) inhibited the enzymatic activity of purified NS3/4A proteases from HCV genotypes 1 to 6 in vitro (IC50: 3.5-11.3 nM) and the replication of stable HCV subgenomic replicons containing proteases from genotypes 1 to 6 (EC50: 0.21-4.6 nM). Glecaprevir had a median EC50 of 0.30 nM (range, 0.05 to 3.8 nM) for HCV replicons containing proteases from 40 samples from patients infected with HCV genotypes 1 to 5 [1]. |
| In vivo | The mean maximal decreases in HCV RNA levels from baseline were approximately 4 log10 IU/ml for all ABT-493 doses ranging from 100 mg to 700 mg and for ABT-530 doses of ≥ 40 mg. There were no meaningful differences in viral load declines for patients with versus without compensated cirrhosis. Twenty-four (50%) of the baseline samples from patients treated with ABT-493 had RAVs to NS3/4A protease inhibitors. Among 40 patients treated with ABT-530, 6 (15%) carried baseline RAVs to NS5A inhibitors [2]. |
| Kinase Assay | Eight recombinant HCV NS3/4A proteases were generated for use in evaluating glecaprevir activity in a biochemical assay. Each recombinant protein contained the entire coding regions of NS3 (amino acids 1 to 631) and NS4A (amino acids 1 to 54) from HCV genotypes 1 to 6, a 6-histidine tag at the N terminus to facilitate purification by affinity chromatography, and three lysine residues at the C terminus to increase the solubility of the protein. Genes encoding NS3/4A were derived from laboratory strains 1a-H77 and 1b-N or from clinical samples from patients infected with genotype 2a, 2b, 3a, or 4a. All patients provided written informed consent. Clinical studies were designed according to Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local regulations, with independent ethics committee or institutional review board approval for all study sites. The genotype 5a NS3/4A gene sequence was synthetically constructed based on the sequence of the clinical isolate SA13, whereas the genotype 6a NS3/4A gene sequence was synthetically constructed based on a consensus sequence derived from the alignment of 15 genotype 6a sequences available in GenBank. The NS3/4A genes were each cloned into the protein expression vector pET14b, and a clone with an NS3/4A protease sequence that matched the consensus sequence for each sample was subsequently selected for protein expression and purification. Protease activity was measured by continuous monitoring of the fluorescence change associated with the cleavage of a fluorogenic depsipeptide (EDANS/DABCYL) substrate using a purified recombinant HCV NS3/4A protease as described previously. The IC50 for each HCV protease was determined in studies in which the protease was preincubated with glecaprevir for 30 min. The percent inhibition was calculated from the initial rates of the inhibited reactions relative to the rate for the uninhibited control [1]. |
| Cell Research | The activity of glecaprevir, paritaprevir, or grazoprevir against cells of nine cell lines each stably transfected with an HCV subgenomic replicon containing NS3 protease from a different HCV genotype was determined using a luciferase reporter assay as described previously. Five of these nine cell lines have been described previously, including those transfected with genotypes 1a H77, 1b Con1, 3a, 4a, and 6a. The other four cell lines were established by transfecting cells with a nonchimeric genotype 2a JFH-1 replicon, two genotype 2a JFH-1 chimeric replicons containing either a genotype 2b NS3 protease domain (N-terminal 251 amino acids) or a sequence encoding full-length NS3 through the first 39 amino acids of NS5B from genotype 5a (strain SA13), and one chimeric replicon with a genotype 1b Con1 backbone containing full-length NS3 and NS4A sequences from genotype 6e. The genotype 2b and 6e NS3 sequences were each synthetically constructed based on a consensus sequence derived from the alignment of 15 genotype 2b and 4 genotype 6e sequences, respectively. All replicon constructs were bicistronic subgenomic replicons similar to those described by Bartenschlager and coworkers, and the replicon cell lines were generated by introducing these constructs into cells of an Huh-7 human hepatoma-derived cell line. The inhibitory effect of the PIs on HCV replication in replicon cells was determined in Dulbecco's modified Eagle medium containing 5% fetal bovine serum with or without 40% human plasma. The EC50s were determined using nonlinear regression curve fitting as described previously [1]. |
| Alias | ABT-493 |
| Molecular Weight | 838.87 |
| Formula | C38H46F4N6O9S |
| Cas No. | 1365970-03-1 |
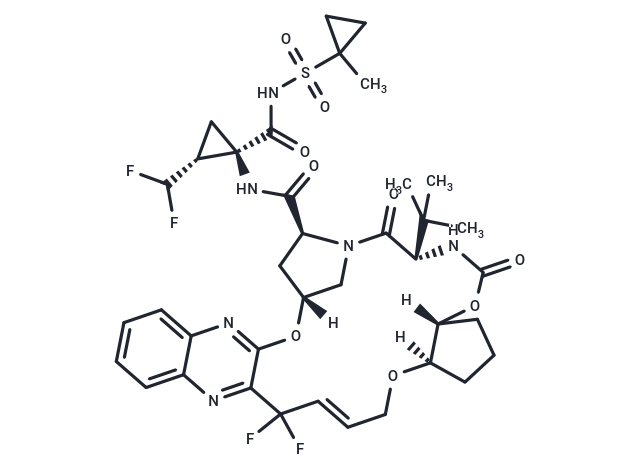
| Smiles | [H][C@@]12C[C@H](N(C1)C(=O)[C@@H](NC(=O)O[C@]1([H])CCC[C@@]1([H])OC\C=C\C(F)(F)c1nc3ccccc3nc1O2)C(C)(C)C)C(=O)N[C@@]1(C[C@H]1C(F)F)C(=O)NS(=O)(=O)C1(C)CC1 |
| Relative Density. | 1.46 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 55 mg/mL (65.56 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.