Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Amsilarotene (TAC101) inhibits the phosphorylation of retinoblastoma gene product (RB) and increases the presence of 2 cyclin-dependent kinases (CDK) inhibitors resulting in cell cycle arrest. This agent also causes a cytotoxic decline in the thymidylate synthase and cyclin A expression.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 mg | $36 | In Stock | |
| 2 mg | $52 | In Stock | |
| 5 mg | $88 | In Stock | |
| 10 mg | $143 | In Stock | |
| 25 mg | $287 | In Stock | |
| 50 mg | $413 | In Stock | |
| 100 mg | $589 | In Stock | |
| 200 mg | $797 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $97 | In Stock |
| Description | Amsilarotene (TAC101) inhibits the phosphorylation of retinoblastoma gene product (RB) and increases the presence of 2 cyclin-dependent kinases (CDK) inhibitors resulting in cell cycle arrest. This agent also causes a cytotoxic decline in the thymidylate synthase and cyclin A expression. |
| Targets&IC50 | RARβ:400 nM (Ki), RARα:2.4 nM (Ki) |
| In vitro | Preclinical models have shown that Amsilarotene(4-[3,5-bis(trimethylsilyl) benzamide] benzoic acid), an oral synthetic retinoid, has antitumor activity in hepatocellular carcinoma (HCC). |
| In vivo | We conducted a phase I study in Japanese patients with advanced HCC to examine the pharmacokinetics, recommended dose, safety, and efficacy of Amsilarotene. The administered dose of Amsilarotene was 10 mg/day in four patients (level 1), 20 mg/day in six (level 2), and 30 mg/day in three (level 3). There was no dose-limiting toxicity at level 1. Only one patient each had dose-limiting toxicity at level 2 (grade 2 fatigue, recovery requiring eight or more consecutive days of rest) and at level 3 (grade 3 splenic vein thrombosis). Level 3 (30 mg/day) was considered the maximum tolerated dose and 20 mg/day the recommended dose by a panel of medical experts, placing maximum emphasis on safety. The most frequent adverse events were fatigue, headache, and dermal symptoms such as rash. Pharmacokinetic parameters in Japanese patients with HCC were similar to those in patients in the United States, most of whom were Caucasian. Although no patient had a complete or partial response, the disease control rate was 38.5%. In conclusion, the recommended dose of Amsilarotene for patients with HCC is 20 mg/day. Amsilarotene had an acceptable toxicity profile, warranting further evaluation in clinical trials. |
| Alias | TAC-101, TAC101, TAC 101 |
| Molecular Weight | 385.6 |
| Formula | C20H27NO3Si2 |
| Cas No. | 125973-56-0 |
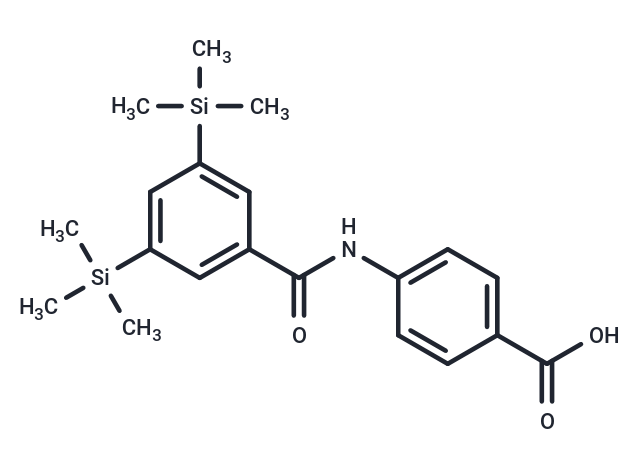
| Smiles | C[Si](C)(C)c1cc(cc(c1)[Si](C)(C)C)C(=O)Nc1ccc(cc1)C(O)=O |
| Relative Density. | 1.103g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 60 mg/mL (155.6 mM), Sonication is recommended. H2O: Insoluble | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.