Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Phenothiazine (ENT 38) is a class of agents exhibiting antiemetic, antipsychotic, antihistaminic, and anticholinergic activities. Phenothiazines antagonize the dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain, potentially preventing chemotherapy-induced emesis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 1 g | $35 | In Stock | |
| 5 g | $80 | In Stock | |
| 10 g | $117 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $30 | In Stock |
| Description | Phenothiazine (ENT 38) is a class of agents exhibiting antiemetic, antipsychotic, antihistaminic, and anticholinergic activities. Phenothiazines antagonize the dopamine D2-receptor in the chemoreceptor trigger zone (CTZ) of the brain, potentially preventing chemotherapy-induced emesis. |
| In vitro | Phenothiazines mostly substitutes at position 10 with the dialkylaminoalkyl groups and additionally at position 2 with small groups exhibit valuable activities such as neuroleptic, antiemetic, antihistaminic, antipuritic, analgesic and antihelmintic. 2-trifluoromethyl-10-(4-aminobutyl)phenothiazine inhibits S. cerevisiae strains and T. mentagrophites with MIC of 0.4 μg/mL and 1.5 μg/mL, respectively. 10-carbamoylalkylphenothiazines shows significant activities against Gram-positive Bacillus subtilis with MIC's in the range of 7.8 μg/mL–30 μg/mL. The tetracyclic phenothiazines (modified with the naphthoquinone ring) shows significant actibacterial activity against S. aureus with the MIC50 of 12.5 μg/mL. Phenothiazines with the butylene linker are more effective than with the propylene linker, the 2-chloro-10-chloroethylureidobutyl derivative giving GI50 of 1.4 μM and 1.6 μM against 4 leukemia cell lines and 7 colon cancer cell lines. 10-Amino(hydroxy)propylphenothiazines (5 μM) induces a marked G2/M phase of cell-cycle arrest followed by cell death in human transformed WI38VA cells after 2-day incubation. [1] Phenothiazine drugs undergo extensive metabolism in the body before being excreted, mainly ring hydroxylation, ring sulphoxidation, N-demethylation, N-oxidation, sulphate and glucuronide conjugation. Phenothiazines have considerably lower binding affinities to α2-adrenoceptors than to dopamine D2 receptors and al-adrenoceptors. [2] Phenothiazines have significant in vitro activity against susceptible, polydrug- and multidrug-resistant strains of M. tuberculosis, as well as enhancing the activity of some agents employed for first-line treatment. [3] |
| Alias | ENT 38 |
| Molecular Weight | 199.27 |
| Formula | C12H9NS |
| Cas No. | 92-84-2 |
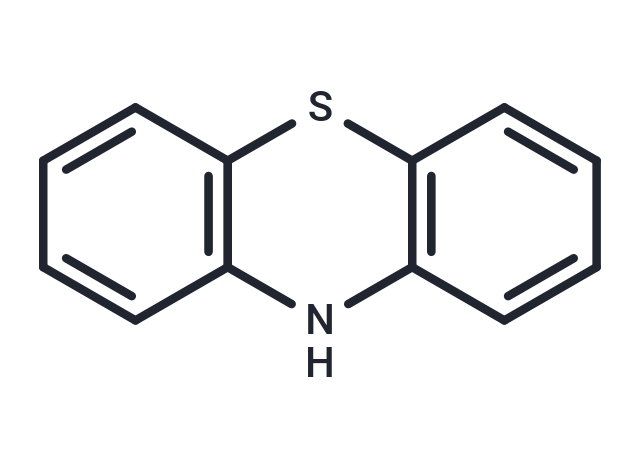
| Smiles | N1C2=C(SC3=C1C=CC=C3)C=CC=C2 |
| Relative Density. | 1.296. Temperature:20 °C. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 45 mg/mL (225.82 mM), Sonication is recommended. Ethanol: < 1 mg/mL (insoluble or slightly soluble) H2O: < 1 mg/mL (insoluble or slightly soluble) | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.