Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

DAPT (LY-374973) is a γ-secretase inhibitor that inhibits total Aβ and Aβ42 (IC50=115/200 nM) and is orally active. DAPT is also a Notch inhibitor. DAPT induces cell differentiation, autophagy and apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $53 | In Stock | |
| 10 mg | $90 | In Stock | |
| 25 mg | $168 | In Stock | |
| 50 mg | $293 | In Stock | |
| 100 mg | $436 | In Stock | |
| 200 mg | $545 | In Stock | |
| 500 mg | $878 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $54 | In Stock |
| Description | DAPT (LY-374973) is a γ-secretase inhibitor that inhibits total Aβ and Aβ42 (IC50=115/200 nM) and is orally active. DAPT is also a Notch inhibitor. DAPT induces cell differentiation, autophagy and apoptosis. |
| Targets&IC50 | β-Amyloid:20 nM (HEK 293 cells), β-Amyloid:115 nM (in human primary neuronal cultures), Aβ42:200 nM (in human primary neuronal cultures) |
| In vitro | METHODS: Ovarian tumor stem cells (OCSC) HO8910 and SKOV3 were treated with DAPT (1-20 μg/mL) for 24-72 h. Cell viability was measured by MTT. RESULTS: A significant decrease in cell self-renewal and proliferation was observed after incubation with DAPT.DAPT induced a concentration-dependent antiproliferative effect on HO8910 and SKOV3 OCSC-like cells. [1] METHODS: Tumor cells GH3 and primary GHoma were treated with DAPT (20-100 nM) for 24 h. Cell migration was monitored using Transwell. RESULTS: DAPT inhibited the migration of GH3 cells and primary GHoma cells. [2] |
| In vivo | METHODS: To test the anti-tumor activity in vivo, DAPT (1-5 mg/kg) was injected intraperitoneally into an athymic immune-deficient nude mouse model harboring rat pituitary tumor GH3 one day a day for fifteen days. RESULTS: DAPT treatment significantly inhibited tumor growth, and the expression of Notch2 and DLL3 was down-regulated in DAPT-treated tumors, with no difference in the expression of DLL4 and VEGF. [2] METHODS: To investigate the effect on cisplatin-induced renal injury, DAPT (15 mg/kg in 20% Captisol) was administered intraperitoneally once daily for five days to a cisplatin-induced renal injury model in C57BL/6J mice. RESULTS: DAPT attenuated cisplatin-induced tubular injury and decreased glomerular filtration rate, and the Notch signaling pathway may be a potential therapeutic target for alleviating cisplatin chemotherapy-associated renal complications. [3] |
| Kinase Assay | water content was 78.83 ± 0.35%. In DAPT group |
| Cell Research | Human embryonic kidney cells, transfected with the gene for APP751 (HEK 293) were used for routine Ab reduction assays. The Ab peptides secreted from these cells have been characterized previously. Cells were plated in 96-well plates and allowed to adhere overnight in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. For compound screening and dose±response testing, compounds were diluted from stock solutions in DMSO to yield a final concentration equal to 0.1% DMSO in media. Cells were pre-treated for 2 h at 378C with compounds, media were aspirated off and fresh compound solutions applied. After an additional 2-h treatment period, conditioned media were drawn off and analyzed by a sandwich ELISA (266±3D6) specific for total Ab. Reduction of Ab production was measured relative to control cells treated with 0.1% DMSO and expressed as percentage inhibition. Data from at least six doses in duplicate were fitted to a four-parameter logistical model using XLfit software in order to determine potency [1]. |
| Animal Research | All studies were conducted with three- to four-month-old heterozygous PDAPP transgenic mice overexpressing the APPV717F a mutant form of the amyloid precursor protein. These animals have been previously shown to exhibit many of the neuropathological features of AD and to produce high levels of Ab in a regionally specific manner. Each treatment group (n=10) consisted of equal numbers of age-matched male and female animals that were fasted overnight prior to treatment. Both treatment and control groups were dosed at a volume of 10 mL/kg with compound formulated in corn oil, 5% (v/v) ethanol or vehicle alone. Tissues were processed and all Ab and APP measurements were made as described previously. After removal of the brain, the cortex from one hemisphere was homogenized, extracted with 5 M guanidine, 50 mM Tris ± pH 8.0, centrifuged, and the supernatant was used for Ab measurements. Cortex from the other hemisphere was snap frozen for analysis of compound levels. Ab levels were expressed as ng/g of wet tissue weight, and percentage reductions were calculated relative to the mean Ab level of tissue from vehicle-treated control animals. Data were analyzed with Mann± Whitney non-parametric statistics to assess significance [1]. |
| Alias | LY-374973, GSI-IX |
| Molecular Weight | 432.46 |
| Formula | C23H26F2N2O4 |
| Cas No. | 208255-80-5 |
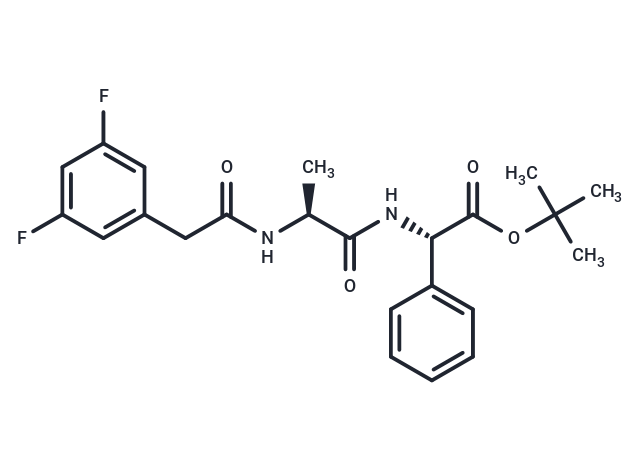
| Smiles | C[C@H](NC(=O)Cc1cc(F)cc(F)c1)C(=O)N[C@H](C(=O)OC(C)(C)C)c1ccccc1 |
| Relative Density. | 1.213g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 4.32 mg/mL (9.99 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. H2O: Insoluble DMSO: 43.2 mg/mL (99.89 mM), Sonication is recommended. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.