Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Osimertinib mesylate (AZD-9291 mesylate) is an EGFR third-generation inhibitor that inhibits the T790M resistance mutation produced by second-generation EGFR inhibitors with irreversible and oral activity. Osimertinib mesylate has antitumor activity for the treatment of EGFR-mutated non-small-cell lung cancer.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $45 | In Stock | |
| 10 mg | $55 | In Stock | |
| 50 mg | $80 | In Stock | |
| 100 mg | $97 | In Stock | |
| 200 mg | $115 | In Stock | |
| 500 mg | $133 | In Stock | |
| 1 g | $155 | In Stock |
| Description | Osimertinib mesylate (AZD-9291 mesylate) is an EGFR third-generation inhibitor that inhibits the T790M resistance mutation produced by second-generation EGFR inhibitors with irreversible and oral activity. Osimertinib mesylate has antitumor activity for the treatment of EGFR-mutated non-small-cell lung cancer. |
| Targets&IC50 | EGFR (L858R):12 nM (cell free), EGFR (L858R/T790M):1 nM (cell free) |
| In vitro | METHODS: Human non-small cell lung cancer cells PC-9 (exon 19del), H3255 (L858R), PC-9ER (exon 19del+T790M) and H1975 (L858R+T790M) were treated with Osimertinib mesylate (0.0001-10 µmol/L) for 72 h, and the growth inhibition of the cells was detected by using MTS method. growth inhibition was detected using the MTS. RESULTS: Osimertinib dose-dependently induced the growth of PC-9, H3255, PC-9ER, and H1975 cells with IC50s of 41, 26, 41, and 31 nM, respectively. [1] METHODS: EGFR-mutated human non-small cell lung cancer cells PC-9, H1975, H1650 and H3255 were treated with Osimertinib mesylate (0.1-1000 nM) for 6 h, and the expression levels of the target proteins were detected by Western Blot. RESULTS: Osimertinib inhibited pEGFR (Y1068), pERK (P-p44/42), and pAKT (S473) in EGFR mutant tumor cells. [2] METHODS: Human non-small cell lung cancer cells NCI-H1975 were treated with Osimertinib mesylate (10-100 nM) for 24 h, then irradiated with 2-20 Gy, and the cell cycle was analyzed by Flow Cytometry. RESULTS: The proportion of G2/M and S-phase cells was dose-dependently reduced in the combination treatment group, and the G2/M-phase cell cycle was arrested after reduced irradiation with Osimertinib. [3] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, Osimertinib mesylate (5-10 mg/kg) was orally administered once daily for seven days to SCID mice bearing human lung cancer tumors H1975, PC9 and A431. RESULTS: Osimertinib treatment significantly inhibited the growth of H1975, PC9 and A431 tumors, indicating antitumor activity in vivo. [4] METHODS: To detect the anti-tumor activity in vivo, Osimertinib mesylate (6 mg/kg) was administered orally to SHO-SCID mice constructed with PC-9/ffluc cells as a model of leptomeningeal carcinomatosis (LMC) once daily for fifty days. RESULTS: Osimertinib treatment significantly delayed the progression of LMC. Osimertinib, a third-generation EGFR-TKI, may be an effective treatment for first- or second-generation EGFR-TKI-resistant LMC caused by EGFR-mutant tumors. [5] |
| Kinase Assay | Kinase assays were performed as per the EMD Millipore profiling service protocol using peptide or protein substrates in a filter-binding radioactive ATP transferase assay for protein [1]. |
| Cell Research | PC-9 cells were seeded into T75 flasks (5 × 10^5 cells/flask) in RPMI growth media and incubated at 37°C, 5% CO2. The following day the media was replaced with media supplemented with a concentration of EGFR inhibitor equal to the EC50 concentration predetermined in PC-9 cells. Media changes were carried out every 2-3 days and resistant clones allowed to grow to 80% confluency prior to the cells being trypsinised and reseeded at the original seeding density in media containing twice the concentration of EGFR inhibitor. Dose escalations were continued until a final concentration of 1.5μM gefitinib, 1.5μM afatinib, 1.5μM WZ4002 or 160nM AZD9291 were achieved [1]. |
| Animal Research | The generation of EGFRL858R and EGFRL858R+T790M mice (male and female) was previously described Doxycycline was administered by feeding mice (aprox 3 week old) with doxycycline-impregnated food pellets (625 ppm) and treated for about 3 months during which time tumors developed. Afatinib and AZD9291 were suspended in 1% Polysorbate 80 and administered via oral gavage once daily at the doses of 7.5 mg/kg and 5 mg/kg, respectively. Mice were imaged weekly at the Vanderbilt University Institute of Imaging Science. For immunoblot analysis, mice were treated for eight hours with drug as described before dissection and flash freezing of the lungs. Lungs were pulverized in liquid nitrogen before lysis as described above [1]. |
| Alias | Mereletinib mesylate, AZD-9291 mesylate |
| Molecular Weight | 595.71 |
| Formula | C29H37N7O5S |
| Cas No. | 1421373-66-1 |
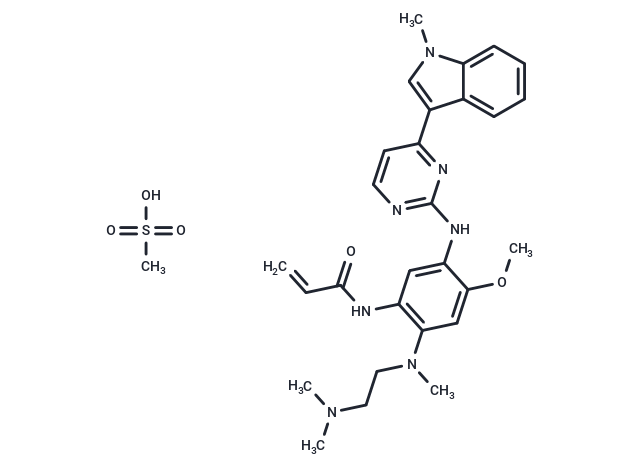
| Smiles | CS(O)(=O)=O.COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 |
| Relative Density. | no data available |
| Storage | store at low temperature,store under nitrogen | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 5.96 mg/mL (10 mM), Sonication is recommended. H2O: 33 mg/mL (55 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO/H2O
H2O
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.