Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Bortezomib (LDP 341) is a 20S proteasome inhibitor (Ki=0.6 nM) that is reversible and selective. Bortezomib has antitumor activity and inhibits NF-κB, which can disrupt the cell cycle and induce apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $48 | In Stock | |
| 10 mg | $58 | In Stock | |
| 25 mg | $73 | In Stock | |
| 50 mg | $88 | In Stock | |
| 100 mg | $147 | In Stock | |
| 200 mg | $237 | In Stock | |
| 500 mg | $392 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $48 | In Stock |
| Description | Bortezomib (LDP 341) is a 20S proteasome inhibitor (Ki=0.6 nM) that is reversible and selective. Bortezomib has antitumor activity and inhibits NF-κB, which can disrupt the cell cycle and induce apoptosis. |
| Targets&IC50 | 20S proteasome:0.6 nM (cell free) |
| In vitro | METHODS: Human tongue squamous carcinoma cells SCC-15 and CAL-27, human pharyngeal squamous carcinoma cells FaDu, and human salivary gland carcinoma cells A-253 and SALTO-5 were treated with Bortezomib (6.25-100 nM) for 24-72 h. The growth inhibition of these cells was detected by SRB. RESULTS: The effects of Bortezomib on the proliferation of the five tumor cells were dose- and time-dependent, and SCC-15 was the most sensitive cell to the effects of Bortezomib. SCC-15 was the most sensitive cell to the effect of Bortezomib.[1] METHODS: Human small cell lung cancer cells NCI-H69 and NCI-H2171 were treated with Bortezomib (0.05 μM; 0.5 μM) for 48 h. Cell cycle and apoptosis were detected by Flow Cytometry. RESULTS: Bortezomib induced cell cycle arrest in the G2-M transition state, increased the number of G2-phase cells and decreased the number of S-phase cells, and induced apoptosis in tumor cells. [2] METHODS: H460, a large cell lung cancer cell, was incubated with Bortezomib (0.01-10 μM) for 3-48 h, and the expression levels of target proteins were detected by Western Blot. RESULTS: Bortezomib treatment resulted in concentration-dependent phosphorylation of Bcl-2 protein. Starting at 12 h, a recognizable Bcl-2 cleavage product was observed, and Bcl-2 phosphorylation preceded Bcl-2 cleavage for at least 9 h.[3] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, Bortezomib (0.3 mg/kg) was administered intraperitoneally to NOD/SCID mice bearing primary exudative lymphoma (PEL) UM-PEL-1 once daily for three weeks. RESULTS: Bortezomib induced remission of PEL and prolonged overall survival of mice with lymphoma exudates. bortezomib downregulated cell cycle progression, DNA replication, and Myc target genes. [4] METHODS: To investigate the effect of Bortezomib on renal fibrosis, Bortezomib (0.5 mg/kg) was intraperitoneally injected into an aristolochic acid I (AA)-induced fibrotic C57BL/6J mouse model twice a week for ten weeks. RESULTS: Bortezomib treatment significantly attenuated AA-induced renal dysfunction and proteinuria, reduced the expression of renal fibrosis-associated proteins and markers of renal injury, such as αSMA, Kim1, and Ngal, and prevented renal fibrosis at histopathologic level. [5] |
| Kinase Assay | Inhibitors were synthesized and purified according to the procedures described in Adams et al.The inhibition constant (Ki) for each inhibitor was measured according to the method of Stein et al.using a fluorometric assay,monitoring peptide substrate cleavage of Z-Leu-Leu-Val-Tyr-amino methyl coumarin (Z = carbobenzyloxy) by the 20S proteasome [1]. |
| Cell Research | PC-3 cells were treated with different doses of PS-341 for different periods of time. The cells were washed with PBS, harvested, and fixed in suspension with 3.7% formaldehyde in the neutral buffer for 10 min at room temperature. The cells were centrifuged, and the cell pellet was resuspended in 0.5 ml of 80% ethanol. The cell suspension (25–50 μl) was then placed onto a microscope slide precoated with poly-l-lysine and air-dried. The slides were washed four times with 0.1% Triton X-100 in PBS. The slide was incubated with the DNA stain Hoechst 33342 (Molecular Probes; 1.0 μg/ml in PBS with 0.1% Triton-X-100) for 1.0 min. The slides were rinsed in PBS and mounted with 70% glycerol containing 25 mg/ml 1,4-diazabicyclo[2.2.2]octane. Nuclear staining was visualized using a fluorescent microscope [1]. |
| Animal Research | Mice were inoculated s.c. into the right flank with 3 × 10^7 MM cells in 100 μl of RPMI 1640, together with 100 μl of Matrigel basement membrane matrix. When tumor was measurable, mice were assigned into four treatment groups receiving PS-341 or into a control group. Treatment with PS-341 was given i.v. twice weekly via tail vein at 0.05, 0.1, 0.5, and 1.0 mg/kg for 4 weeks. Subsequently, it was administered once weekly. The control group received the vehicle alone (0.9% sodium chloride) at the same schedule. Caliper measurements of the longest perpendicular tumor diameters were performed every alternate day to estimate the tumor volume, using the following formula: 4π/3 × (width/2)^2 × (length/2), representing the three-dimensional volume of an ellipse. Animals were sacrificed when their tumors reached 2 cm or when the mice became moribund. Survival was evaluated from the first day of treatment until death [4]. |
| Alias | Radiciol, NSC 681239, MG 341, LDP 341, DPBA, Brotezamide |
| Molecular Weight | 384.24 |
| Formula | C19H25BN4O4 |
| Cas No. | 179324-69-7 |
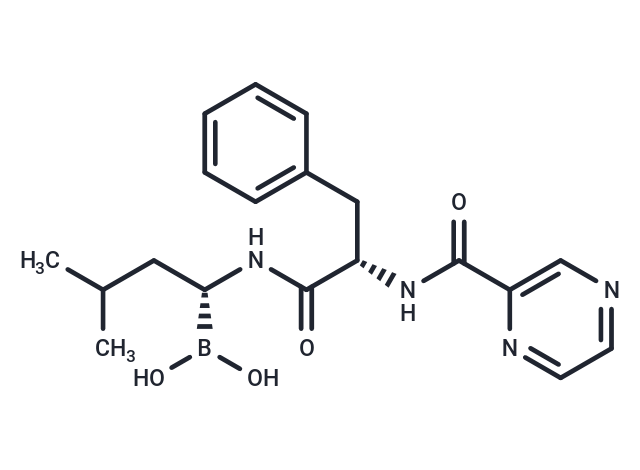
| Smiles | CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |
| Relative Density. | 1.214 |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 7.1 mg/mL (18.48 mM), In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. H2O: Insoluble Ethanol: 20.83 mg/ml (54.21 mM), Sonication is recommended. DMSO: 71 mg/mL (184.78 mM), Sonication is recommended. | |||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| ||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.