Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Ulipristal acetate (CDB-2914) is an orally bioavailable selective progesterone receptor modulator with anti-progesterone activity. It binds to the progesterone receptor (PR), inhibiting PR-mediated gene expression and interfering with progesterone activity in the reproductive system, potentially suppressing the growth of uterine leiomyomatosis. Additionally, ulipristal can be used as emergency contraception by inhibiting or delaying ovulation and affecting endometrial tissue.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $45 | In Stock | |
| 10 mg | $61 | In Stock | |
| 50 mg | $126 | In Stock | |
| 100 mg | $198 | In Stock | |
| 200 mg | $297 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $51 | In Stock |
| Description | Ulipristal acetate (CDB-2914) is an orally bioavailable selective progesterone receptor modulator with anti-progesterone activity. It binds to the progesterone receptor (PR), inhibiting PR-mediated gene expression and interfering with progesterone activity in the reproductive system, potentially suppressing the growth of uterine leiomyomatosis. Additionally, ulipristal can be used as emergency contraception by inhibiting or delaying ovulation and affecting endometrial tissue. |
| In vitro | Ulipristal acetate blocks activin A modulation of fibronectin and vascular endothelial growth factor A (VEGF-A) mRNA expression in cultured myometrial and leiomyoma cells[2]. Ulipristal acetate decreases the DNA fragmentation at the 100-ng/mL dose and continuing up to the 10,000-ng/mL dose compared to those spermatozoa in the control group[3]. |
| In vivo | Ulipristal and CDB-4124 exhibit notable antiprogestational effects in vivo[1]. Ulipristal acetate has been shown to reduce the occurrence of fibroadenomas and adenocarcinomas in the mammary glands across all groups studied. At the highest administered dose in rats, ulipristal acetate exposure [AUC(0-24h)] is 67 times the human therapeutic exposure at 10 mg/day. Importantly, in mice, ulipristal acetate does not lead to an increase in tumor formation, even at exposures up to 313 times the therapeutic level. Adverse effects in mice are confined to weight changes in specific organs (liver, pituitary, thyroid/parathyroid glands, and epididymis) and minimal panlobular hepatocellular hypertrophy at a dose of 130 mg/kg/day[4]. Additionally, ulipristal acetate at doses of 1 mg/kg and 5 mg/kg causes a dose-dependent increase in endometrial thickening, observed by pathologists more frequently than in controls. There is also a minor decrease in secretory differentiation as the dose of ulipristal acetate increases, indicated by reduced sub- and supra-nuclear vacuolation[5]. |
| Alias | Ulipristal, CDB-2914 |
| Molecular Weight | 475.62 |
| Formula | C30H37NO4 |
| Cas No. | 126784-99-4 |
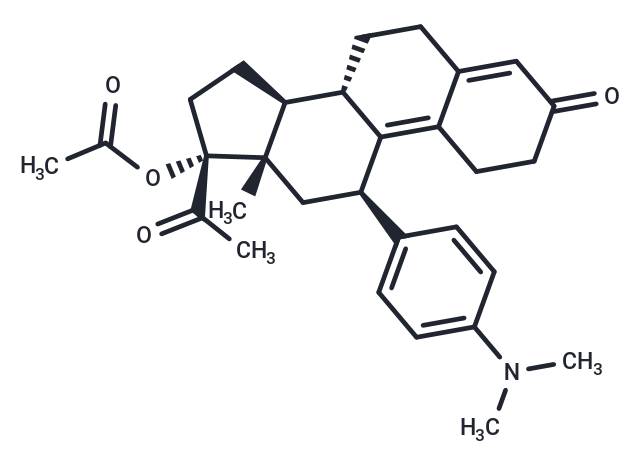
| Smiles | CN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@]2(OC(C)=O)C(C)=O)[C@@H]2CCC3=CC(=O)CCC3=C12 |
| Relative Density. | 1.19 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (105.13 mM), Sonication is recommended. | |||||||||||||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.