Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Doxorubicin hydrochloride (Adriamycin) belongs to the anthracycline class of antibiotics and is an inhibitor of human DNA topoisomerase I/II (IC50=0.8/2.67 μM). Doxorubicin hydrochloride exhibits cytotoxicity and antitumor activity. Doxorubicin hydrochloride reduces the phosphorylation of AMPK and its downstream target protein acetyl coenzyme A carboxylase, and induces apoptosis and autophagy.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $34 | In Stock | |
| 50 mg | $48 | In Stock | |
| 100 mg | $68 | In Stock | |
| 200 mg | $98 | In Stock | |
| 500 mg | $187 | In Stock | |
| 1 g | $276 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock |
| Description | Doxorubicin hydrochloride (Adriamycin) belongs to the anthracycline class of antibiotics and is an inhibitor of human DNA topoisomerase I/II (IC50=0.8/2.67 μM). Doxorubicin hydrochloride exhibits cytotoxicity and antitumor activity. Doxorubicin hydrochloride reduces the phosphorylation of AMPK and its downstream target protein acetyl coenzyme A carboxylase, and induces apoptosis and autophagy. |
| Targets&IC50 | Topo I:0.8 μM (IC50), Topo II:2.67 μM (IC50) |
| In vitro | METHODS: Human breast cancer cells MCF10A, BT474, MCF-7 and T47D were treated with Doxorubicin hydrochloride (0.1-10 μM) for 48 h, and cell growth inhibition was detected by MTT. RESULTS: Doxorubicin hydrochloride dose-dependently inhibited the growth of MCF10A, BT474, MCF-7 and T47D cells with IC50s of 2.51 µM, 1.14 µM, 0.69 µM and 8.53 µM, respectively. [1] METHODS: Bovine aortic endothelial cells BAECs and human ovarian teratoma cells PA-1 were treated with Doxorubicin (0.5 μM) for 1-16 h. Apoptosis was detected by Flow Cytometry, and caspase-3 activity was detected by caspase-3 assay kit. RESULTS: Doxorubicin induced apoptosis and caspase-3 activation in BAECs and PA-1 cells in a time-dependent manner. [2] METHODS: Canine breast cancer cells CIPp were treated with Doxorubicin (EC50(20h)=12.08 μM) for 3-48 h. The expression of target genes was detected by qRT-PCR. RESULTS: Doxorubicin induced up-regulation of the mRNA expression levels of multidrug resistance (MDR)-related genes P-gp and BCRP. [3] |
| In vivo | METHODS: To detect antitumor activity in vivo, Doxorubicin hydrochloride (1 mg/kg/4 days) and lovastatin (5 mg/kg/day) were intraperitoneally injected into B6D2F1 mice bearing murine melanoma tumor B16F10 for two weeks. RESULTS: The combination of Doxorubicin hydrochloride and lovastatin showed a significant increase in sensitivity compared to either drug alone. lovastatin enhanced the antitumor activity of Doxorubicin hydrochloride. [4] METHODS: To investigate the acute and long-term cognitive deficits of Doxorubicin in cancer patients, a single dose of Doxorubicin hydrochloride (25 mg/kg) was administered intraperitoneally to B6C3F1J mice. RESULTS: Systemic treatment with Doxorubicin hydrochloride altered glutamatergic neurotransmission in the nucleus of key cells associated with cognitive function within 24 h. There were no lasting effects on spatial learning and memory. [5] |
| Cell Research | To analyze the effect of Bcl-2 expression on the viability of HUVECs treated with Dox, cells were co-transfected with 200 ng of the pEGFP-spectrin expression plasmid together with 200 ng of either pCDNA3-hBcl-2 or the control pCMVβ-galactosidase expression vector (33). The pGL3 Basic vector (2.1 μg) was added as a DNA carrier in a total volume of 0.140 ml, and transfection was performed by the calcium phosphate procedure in 35-mm tissue culture dishes. After treatment, the cells were washed with PBS, fixed with 3.7% formaldehyde for 15 min, and washed for a further 10 min with 50 mM NH4Cl blocking solution in PBS. Cells were then washed with PBS, permeabilized with a 0.1% Triton X-100 for 10 min, washed again with PBS, and stained with 1 μg/ml 4′,6-diamidino-2-phenyl-indole solution for 2 min. The cells were examined under a fluorescence microscope, and GFP-positive cells were scored after counting a minimum of 1000 total cells for each condition. The efficiency of transfection in Bcl-2- and β-galactosidase-expressing cells, determined in aliquots of transfected cells just before the addition of Dox, was similar (10–12%) [1]. |
| Animal Research | Athymic male nude mice (3-4 weeks old) are used. PC3 cells (4×106) are injected subcutaneously into the flanks of mice. Animals bearing tumors are randomly assigned to treatment groups (five or six mice per group) and treatment initiated when xenografts reached volumes of about 100 mm3. Tumors are measured using digital calipers and volume calculated using the formula: Volume=Width2×Length×0.52, where width represents the shorter dimension of the tumor. Treatments are administered as indicated using vehicle (PBS containing 0.1% BSA), Doxorubicin (2-8 mg/kg), Apo2L/TRAIL (500 μg/animal), or a combination of 4 mg/kg Doxorubicin followed by 500 μg Apo2L/TRAIL. Doxorubicin is administered systemically whereas Apo2L/TRAIL is given either intratumorally or systemically. All treatments are given once. Mice are monitored daily for signs of adverse effects (listlessness and scruffy appearance). Treatments seemed to be well tolerated. The mean±SEM is calculated for each data point. Differences between treatment groups are analyzed by the student t-test. Differences are considered significant when P<0.05 [3]. Altogether, 29 male Wistar rats (weight 306 ± 18.6 g) were used in the study. Animals were divided into three groups: control (group C; n = 10; 306.4 ± 17.2 g), animals treated with DOX (group DOX; n = 10; 305.0 ± 24.9 g) and animals treated with L-DOX (group L-DOX; n = 9; 306.7 ± 15.0 g). Vehiculum (aqua pro injection), DOX and L-DOX were applied to group C, DOX and L-DOX, respectively, by single intraperitoneal injection; concentration of both DOX and L-DOX was 5 mg/kg, similar to the concentrations used in human treatment protocols. All animals were sacrificed 24 h after drug application. Thoracotomy was performed, hearts were excised and samples were obtained separately from the free wall of the left atrium (LA), left ventricle (LV), right atrium (RA) and right ventricle (RV).Samples were placed into RNA later preservation solution and stored at -80 C until further analysis [4]. |
| Alias | NSC 123127, Hydroxydaunorubicin hydrochloride, DOX hydrochloride, Adriamycin HCl |
| Molecular Weight | 579.99 |
| Formula | C27H29NO11·HCl |
| Cas No. | 25316-40-9 |
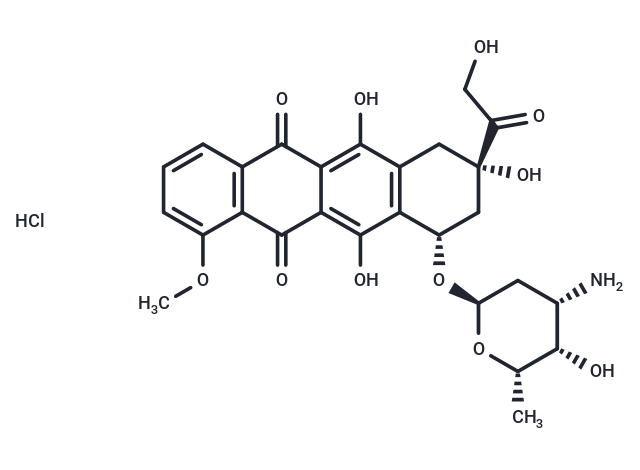
| Smiles | Cl.COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 55 mg/mL (94.83 mM), Sonication is recommended. H2O: 50 mg/mL (86.2 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
H2O/DMSO
| |||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.