Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Fluoxetine (LY-110140) is a highly specific serotonin uptake inhibitor and selective 5-hydroxytryptamine (5-HT) reuptake inhibitor. Fluoxetine has antidepressant activity.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 10 mg | $30 | In Stock | |
| 25 mg | $41 | In Stock | |
| 50 mg | $57 | In Stock | |
| 100 mg | $74 | In Stock | |
| 200 mg | $123 | In Stock | |
| 500 mg | $225 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $39 | In Stock |
| Description | Fluoxetine (LY-110140) is a highly specific serotonin uptake inhibitor and selective 5-hydroxytryptamine (5-HT) reuptake inhibitor. Fluoxetine has antidepressant activity. |
| In vitro | METHODS: Mouse cortical near-pure neuronal cell cultures were treated with Fluoxetine (3-30 µM) for 24 h. Cell viability was measured by MTT assay. RESULTS: Fluoxetine induced neuronal death in a concentration-dependent manner. [1] METHODS: Human gastric cancer cells AGS were treated with Fluoxetine (10-20 µM) for 24 h. Apoptosis was detected by Flow cytometry. RESULTS: The number of early apoptotic cells in Fluoxetine-treated group was significantly increased by about 4-fold and 10-fold, respectively. [2] |
| In vivo | METHODS: To assay antidepressant activity in vivo, Fluoxetine (2.5-10 mg/kg) was administered intraperitoneally to MRL/MpJ mice twice daily for twenty-six days. RESULTS: Chronic treatment with 5 and 10 mg/kg Fluoxetine significantly increased cell proliferation and BDNF levels in the hippocampus. Only chronic treatment with the highest Fluoxetine increased BDNF levels in the frontal cortex. [3] METHODS: To test antidepressant activity in vivo, Fluoxetine (18 mg/kg) was administered orally once daily for three weeks to a model of corticosterone-induced anxiety/depression-like behavior in C57BL/6Ntac mice. RESULTS: Chronic Fluoxetine treatment reversed the anxiety phenotype. Regarding total distance traveled, chronic corticosterone treatment showed a nonsignificant trend that was eliminated by chronic Fluoxetine treatment. [4] |
| Alias | LY-110140 |
| Molecular Weight | 309.33 |
| Formula | C17H18F3NO |
| Cas No. | 54910-89-3 |
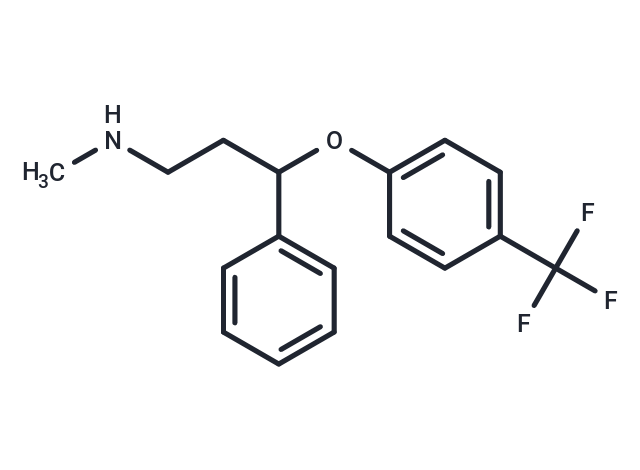
| Smiles | CNCCC(Oc1ccc(cc1)C(F)(F)F)c1ccccc1 |
| Relative Density. | 1.159 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||
| Solubility Information | DMSO: 10 mg/mL (32.33 mM), Sonication is recommended. | |||||||||||||||||||||||||
| In Vivo Formulation | 5% DMSO+95% Saline: 0.3 mg/mL (0.97 mM), In vivo: Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. The formulation provided above is for reference purposes only. In vivo formulations may vary and should be modified based on specific experimental conditions. | |||||||||||||||||||||||||
Solution Preparation Table | ||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.