- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Halobetasol propionate
Halobetasol propionate (BMY-30056) is the propionate salt form of halobetasol, a synthetic corticosteroid with anti-inflammatory, antipruritic, and vasoconstrictor activities. Halobetasol, a topical steroid, diffuses across cell membranes to interact with cytoplasmic corticosteroid receptors located in both the dermal and intradermal cells, thereby activating gene expression of anti-inflammatory proteins mediated via corticosteroid receptor response element. Specifically, this agent induces phospholipase A2 inhibitory proteins, which inhibit the release of arachidonic acid, thereby inhibiting the biosynthesis of potent mediators of inflammation, such as prostaglandins and leukotrienes. As a result, halobetasol reduces edema, erythema, and pruritus through its cutaneous effects on vascular dilation and permeability.
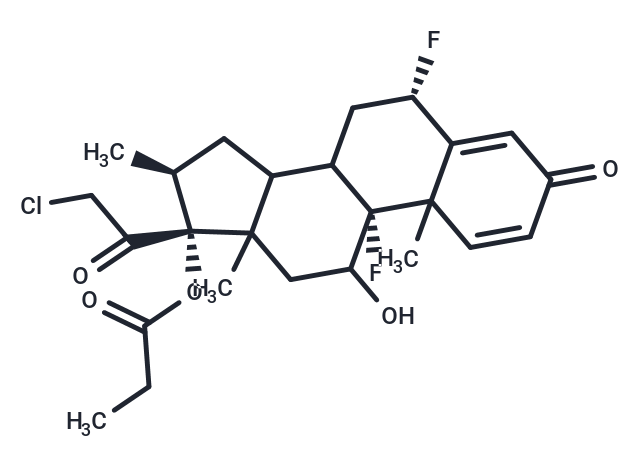
Halobetasol propionate
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 25 mg | $35 | In Stock | |
| 50 mg | $44 | In Stock | |
| 100 mg | $70 | In Stock | |
| 200 mg | $98 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $29 | In Stock |
Product Introduction
| Description | Halobetasol propionate (BMY-30056) is the propionate salt form of halobetasol, a synthetic corticosteroid with anti-inflammatory, antipruritic, and vasoconstrictor activities. Halobetasol, a topical steroid, diffuses across cell membranes to interact with cytoplasmic corticosteroid receptors located in both the dermal and intradermal cells, thereby activating gene expression of anti-inflammatory proteins mediated via corticosteroid receptor response element. Specifically, this agent induces phospholipase A2 inhibitory proteins, which inhibit the release of arachidonic acid, thereby inhibiting the biosynthesis of potent mediators of inflammation, such as prostaglandins and leukotrienes. As a result, halobetasol reduces edema, erythema, and pruritus through its cutaneous effects on vascular dilation and permeability. |
| In vitro | Halobetasol propionate is thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. The initial interaction, however, is due to the drug binding to the cytosolic glucocorticoid receptor. After binding the receptor the newly formed receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing the increase in expression of specific target genes.[1] |
| Alias | Ulobetasol propionate, Halobetasol Propionate, CGP-14458, BMY-30056 |
| Molecular Weight | 484.96 |
| Formula | C25H31ClF2O5 |
| Cas No. | 66852-54-8 |
| Smiles | CCC(=O)O[C@@]1([C@@H](C)CC2C3C[C@H](F)C4=CC(=O)C=CC4(C)[C@@]3(F)C(O)CC12C)C(=O)CCl |
| Relative Density. | 1.31 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: < 1 mg/mL (insoluble or slightly soluble) Ethanol: 36 mg/mL (74.23 mM), Sonication is recommended. DMSO: 90 mg/mL (185.58 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
Ethanol/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
Calculator
In Vivo Formulation Calculator (Clear solution)
Dose Conversion
Tech Support
Keywords

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



