Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Nilotinib (AMN107) is a Bcr-Abl tyrosine kinase inhibitor with oral activity. Nilotinib has antitumor activity and may be used for the treatment of Imatinib-resistant chronic myelogenous leukemia (CML).
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 50 mg | $35 | In Stock | |
| 100 mg | $42 | In Stock | |
| 200 mg | $50 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock |
| Description | Nilotinib (AMN107) is a Bcr-Abl tyrosine kinase inhibitor with oral activity. Nilotinib has antitumor activity and may be used for the treatment of Imatinib-resistant chronic myelogenous leukemia (CML). |
| Targets&IC50 | Abl (WT):15 nM (cell free) |
| In vitro | METHODS: Ba/F3 cells expressing wild-type or mutant Bcr-Abl were treated with Nilotinib for 72 h, and cell viability was measured by methanethiosulfonate-based viability assay. RESULTS: Nilotinib inhibited the growth of cells expressing wild-type Bcr-Abl with 20-fold higher potency than imatinib (IC50:13 vs. 260 nmol/L). Similar improvements were maintained in all imatinib-resistant mutants tested except T315I. [1] METHODS: Melanoma cell line D04 was treated with Nilotinib (0.1-10 µM) for 3 h. Target protein expression levels were examined by Western Blot. RESULTS: Nilotinib stimulated robust MEK and ERK phosphorylation at concentrations as low as 100 nM. [2] |
| In vivo | METHODS: To test the antitumor activity in vivo, Nilotinib (25 mg/kg) and PD184352 (25 mg/kg) were administered by gavage to Balb/cJ mice bearing Ba/F3 allografts of BCR-ABL or BCR-ABLT315I once daily for twenty days. RESULTS: Nilotinib strongly inhibited the growth of BCR-ABL tumors, but not PD184352, nor did PD184352 enhance the growth inhibitory activity of Nilotinib. In contrast, BCR-ABLT315I tumors were insensitive to both Nilotinib and PD184352, but these drugs synergistically inhibited tumor growth. [2] |
| Kinase Assay | Kinase assays using wild-type and mutant glutathione S-transferase (GST)–Abl fusion proteins (c-Abl amino acids 220-498) were done as described, with minor alterations. GST-Abl fusion proteins were released from glutathione-Sepharose beads before use; the concentration of ATP was 5 μmol/L. Immediately before use in kinase autophosphorylation and in vitro peptide substrate phosphorylation assays, GST-Abl kinase domain fusion proteins were treated with LAR tyrosine phosphatase according to the manufacturer's instructions. After 1-hour incubation at 30°C, LAR phosphatase was inactivated by addition of sodium vanadate (1 mmol/L). Immunoblot analysis comparing untreated GST-Abl kinase to dephosphorylated GST-Abl kinase was routinely done using phosphotyrosine-specific antibody 4G10 to confirm complete (>95%) dephosphorylation of tyrosine residues and c-Abl antibody CST 2862 to confirm equal loading of GST-Abl kinase. The inhibitor concentration ranges for IC50 determinations were 0 to 5,000 nmol/L (imatinib and AMN107) or 0 to 32 nmol/L (BMS-354825). The BMS-354825 concentration range was extended to 1,000 nmol/L for mutant T315I. These same inhibitor concentrations were used for the in vitro peptide substrate phosphorylation assays. The three inhibitors were tested over these same concentration ranges against GST-Src kinase and GST-Lyn kinase [1]. |
| Cell Research | Ba/F3 cell lines were plated in triplicate and incubated with escalating concentrations of imatinib, AMN107, or BMS-354825 for 72 hours. Proliferation was measured using a methanethiosulfonate-based viability assay. IC50 and IC90 values are reported as the mean of three independent experiments done in quadruplicate. The inhibitor concentration ranges for IC50 and IC90 determinations were 0 to 2,000 nmol/L (imatinib and AMN107) or 0 to 32 nmol/L (BMS-354825). The imatinib concentration range was extended to 6,400 nmol/L for mutants with IC50 >2,000 nmol/L. The BMS-354825 concentration range was extended to 200 nmol/L for mutant T315I [1]. |
| Animal Research | The GIST xenograft lines GK1X, GK2X and GK3X in nude mice were established from GIST patients as described in our previous study [10]. These xenograft lines were maintained by continual passage in BALB/cSLc-nu/nu mice. Mice bearing GK1X, GK2X and GK3X tumors (6–8 mice per group) were treated daily with vehicle or 40 mg/kg imatinib or nilotinib for 4 weeks. Tumor volume (TV) was determined from caliper measurements of tumor length (L) and width (w) according to the formula LW2/2. TV was determined every two to three days and on the day of evaluation. Mice were sacrificed and the percentage of tumor growth inhibition (TGI) was calculated as follows: TGI (%) ?=? [1– (mean of treatment group tumor volume on evaluation day – mean of treatment group tumor volume on day 1)/(mean of control group tumor volume on evaluation day – mean of control group tumor volume on day 1)]×100 [2]. |
| Alias | Tasigna, AMN107 |
| Molecular Weight | 529.52 |
| Formula | C28H22F3N7O |
| Cas No. | 641571-10-0 |
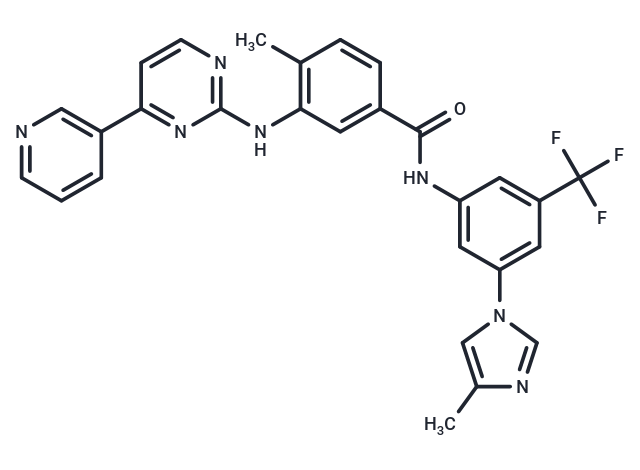
| Smiles | C(F)(F)(F)C=1C=C(C=C(NC(=O)C2=CC(NC=3N=C(C=CN3)C=4C=CC=NC4)=C(C)C=C2)C1)N5C=NC(C)=C5 |
| Relative Density. | 1.36 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | H2O: < 1 mg/mL (insoluble or slightly soluble) 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 2.6 mg/mL (4.91 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. Ethanol: < 1 mg/mL (insoluble or slightly soluble) DMSO: 13.75 mg/mL (25.97 mM), Sonication is recommended. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.