Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

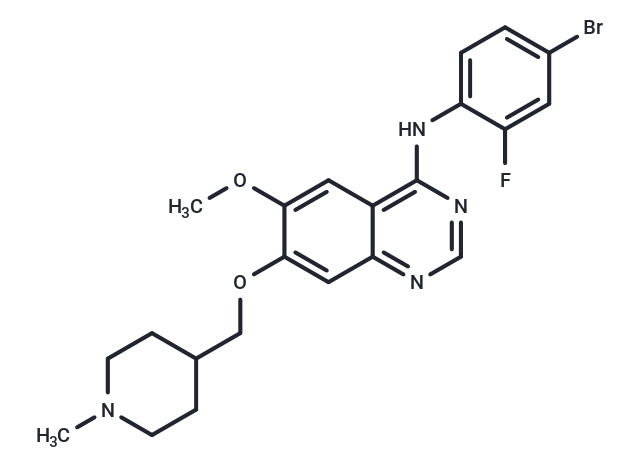
Vandetanib (ZD6474) is a potent inhibitor of VEGFR2 (IC50: 40 nM). It also inhibits VEGFR3 and EGFR.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 10 mg | $45 | In Stock | |
| 25 mg | $63 | In Stock | |
| 50 mg | $98 | In Stock | |
| 100 mg | $178 | In Stock | |
| 200 mg | $248 | In Stock | |
| 500 mg | $398 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock |
| Description | Vandetanib (ZD6474) is a potent inhibitor of VEGFR2 (IC50: 40 nM). It also inhibits VEGFR3 and EGFR. |
| Targets&IC50 | VEGFR2:40 nM (cell free), VEGFR3:110 nM (cell free), EGFR:500 nM (cell free) |
| In vitro | Vandetanib (ZD6474) is a potent inhibitor of KDR/VEGFR 2 tyrosine kinase activity (IC50: 40 nM). This compound has some additional activity versus the tyrosine kinase activity of VEGFR3 (IC50: 110 nM) and EGFR/HER1 (IC50: 500 nM). The activity of ZD6474 versus KDR tyrosine kinase translates into potent inhibition of VEGF-stimulated endothelial cell (human umbilical vein endothelial cell) proliferation in vitro (IC50: 60 nM) [1]. ZD6474 causes a dose-dependent inhibition of EGFR phosphorylation in mouse NIH-EGFR fibroblasts and human MCF-10A ras breast cancer cells. ZD6474 treatment resulted in a dose-dependent inhibition of soft agar growth in seven human cell lines with functional EGFR but lacking VEGFR-2. A dose-dependent supra-additive effect in growth inhibition and in apoptosis in vitro was observed by the combined treatment with ZD6474 and paclitaxel or docetaxel [2]. Vandetanib and neratinib displayed an inhibitory effect on the basal ABCG2-ATPase. At relatively high concentrations (10–20 mM), vandetanib inhibited the stimulated ABCG2-ATPase [3]. |
| In vivo | Administration of ZD6474 (2.5 mg/kg, i.v.) reversed a hypotensive change induced by VEGF (by 63%) but did not significantly affect that induced by basic fibroblast growth factor. Administration of 50 mg/kg/day ZD6474 (once-daily, p.o.) to athymic mice with intradermally implanted A549 tumor cells also inhibited tumor-induced neovascularization significantly (63% inhibition after 5 days). Histological analysis of Calu-6 tumors treated with 50 mg/kg/day ZD6474 for 24 days showed a significant reduction (>70%) in CD31 (endothelial cell) staining in nonnecrotic regions [1]. ZD6474 treatment of nude mice bearing palpable GEO colon cancer xenografts induced dose-dependent tumor growth inhibition. The antitumor activity of ZD6474 in GEO tumor xenografts was also found to be enhanced when combined with paclitaxel. Tumor regression was observed in all mice after treatment with ZD6474 plus paclitaxel, and it was accompanied by a significant potentiation in the inhibition of angiogenesis [2]. Vandetanib (15 mg/kg) had similar effects on the growth of H1650/PTEN and H1650 parental xenograft tumors [4]. |
| Kinase Assay | The ability of ZD6474 to inhibit the kinase activity associated with the VEGFRs KDR, Flt-1, and Flt-4 was determined using a previously described ELISA. Briefly, ZD6474 was incubated with enzyme, 10 mm MnCl2, and 2 μm ATP in 96-well plates coated with a poly(Glu, Ala, Tyr) 6:3:1 random copolymer substrate. Phosphorylated tyrosine was then detected by sequential incubation with a mouse IgG anti-phosphotyrosine 4G10 antibody, a horseradish peroxidase-linked sheep anti-mouse immunoglobulin antibody, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid). Microcal Origin software was used to interpolate IC50 values by nonlinear regression. This methodology was adapted to examine selectivity versus tyrosine kinases associated with EGFR, PDGFRβ, Tie-2, FGFR1, c-kit, erbB2, IGF-IR, and FAK. All enzyme assays (tyrosine or serine-threonine) used appropriate ATP concentrations at or just below the respective Km (0.2–14 μm). Selectivity versus serine-threonine kinases (CDK2, AKT, and PDK1) was examined using a relevant scintillation proximity assay (SPA) in 96-well plates. CDK2 assays contained 10 mm MnCl2, 4.5 μm ATP, 0.15 μCi of [γ-33P]ATP/reaction, 50 mm HEPES (pH 7.5), 1 mm DTT, 0.1 mm sodium orthovanadate, 0.1 mm sodium fluoride, 10 mm sodium glycerophosphate, 1 mg/ml BSA fraction V, and a retinoblastoma substrate (part of the retinoblastoma gene, 792–928, expressed in a glutathione S-transferase expression system; 0.22 μm final concentration). Reactions were allowed to proceed at room temperature for 60 min before quenching for 2 h with 150 μl of a solution containing EDTA (62 mm final concentration), 3 μg of a rabbit immunoglobulin anti-glutathione S-transferase antibody and protein A SPA-polyvinyltoluene beads (0.8 mg/reaction). Plates were then sealed, centrifuged (1200 × g for 5 min), and counted on a Topcount NXT Microplate scintillation counter for 30 s [1]. |
| Cell Research | HUVEC proliferation in the presence and absence of growth factors was evaluated using [3H]thymidine incorporation. Briefly, HUVECs isolated from umbilical cords were plated (at passage 2–8) in 96-well plates (1000 cells/well) and dosed with ZD6474 ± VEGF or EGF (3 ng/ml) or bFGF (0.3 ng/ml). The cultures were incubated for 4 days (37°C; 7.5% CO2) and then pulsed with 1 μCi/well [3H]thymidine and reincubated for 4 h. Cells were harvested and assayed for the incorporation of tritium using a beta counter. IC50 data were interpolated as described above [1]. |
| Animal Research | Methodology to enable blood pressure measurement in anesthetized rats was as described previously. Briefly, anesthesia was induced in male Alderley Park rats using α-chloralose by the i.v. route and then maintained with thiopentone via the i.p. route. Once surgical anesthesia was established, the carotid artery was cannulated to enable blood pressure recording using a pressure transducer and a Lectromed MT8P amplifier. The jugular vein was cannulated to allow growth factor administration. Body temperature was maintained with a thermostatically controlled heated blanket coupled to a rectal thermometer. Human VEGF165 (32 μg/kg) or bFGF (40 μg/kg) was administered as a bolus injection [0.1 ml/250 g body weight in 0.85% (w/v) sodium chloride], and a maximal blood pressure drop was recorded within 2 min (typically 26–30 mm Hg). These changes were sustainable for more than 20 min in control experiments. ZD6474 (2.5 mg/kg) or vehicle alone [25% (w/v) hydroxypropyl-β-cyclodextrin in Sorensons phosphate buffer (pH 5.5)] was administered i.v., and blood pressure was recorded 5 min later to determine the effect on growth factor-induced hypotension [1]. |
| Alias | ZD6474 |
| Molecular Weight | 475.35 |
| Formula | C22H24BrFN4O2 |
| Cas No. | 443913-73-3 |
| Smiles | COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 |
| Relative Density. | 1.406 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 27.5 mg/mL (57.85 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||
DMSO
| |||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.