Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

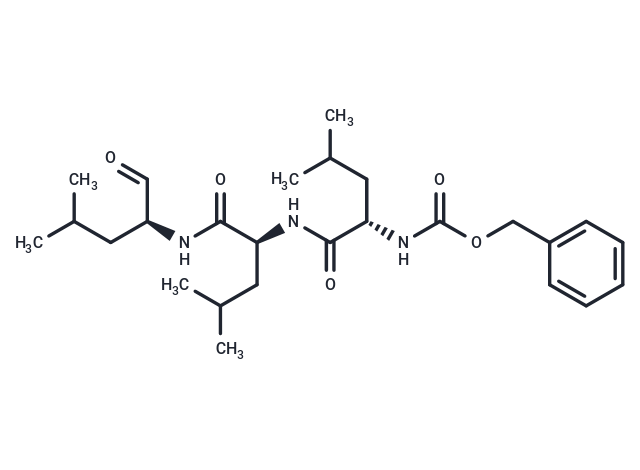
MG-132 (Z-Leu-Leu-Leu-al) is a 26S proteasome inhibitor (IC50=100 nM) that is cell-permeable and reversible. MG-132 acts as an autophagy activator and also induces apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 10 mg | $40 | In Stock | |
| 25 mg | $81 | In Stock | |
| 50 mg | $147 | In Stock | |
| 100 mg | $228 | In Stock | |
| 200 mg | $396 | In Stock | |
| 500 mg | $497 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $39 | In Stock |
| Description | MG-132 (Z-Leu-Leu-Leu-al) is a 26S proteasome inhibitor (IC50=100 nM) that is cell-permeable and reversible. MG-132 acts as an autophagy activator and also induces apoptosis. |
| Targets&IC50 | 20S proteasome:100 nM (cell free), Calpain:1.2 μM (cell free) |
| In vitro | METHODS: Human cervical cancer cells HeLa were treated with MG-132 (0.5-30 μM) for 24 h, and cell growth inhibition was detected by MTT. RESULTS MG-132 dose-dependently inhibited HeLa cell growth with an IC50 of approximately 5 μM. [1] METHODS: Human mesothelioma cells NCI-H2452 were treated with MG-132 (0.25-2 μM) for 36 h, and the expression levels of target proteins were detected by Western Blot. RESULTS MG-132 treatment induces cleavage of caspases 3 and 7, Bid, and PARP in NCI-H2452 cells. MG-132 induces a caspase-dependent apoptosis. [2] METHODS: Human melanoma cells MeWo were treated with MG-132 (0.01-1 μM) for 24 h, and the cell cycle profiles were analyzed by Flow Cytometry. RESULTS MG-132 induces cell cycle arrest at G2 phase in MeWo cells. [3] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, MG-132 (1 mg/kg) was injected intravenously into C.B-17/lcr-scid/scidJcl mice harboring the human cervical cancer tumors HeLa, CaSki, or C33A twice a week for 4 weeks. RESULTS MG-132 significantly inhibited the growth of human cervical cancer tumors, indicating antitumor activity in vivo. [4] METHODS: To investigate the effects of long-term treatment with MG-132 on cardiac hypertrophy and its associated molecular mechanisms, MG-132 (0.1 mg/kg) was injected intraperitoneally into rats with an abdominal aortic band (AAB) once daily for 8 weeks. RESULTS MG-132 treatment significantly attenuated left ventricular myocyte area, left ventricular weight/body weight, and lung weight/body weight ratios, decreased left ventricular diastolic diameter and wall thickness, and increased the shortening fraction in AAB rats. MG-132 treatment significantly reversed the elevated levels of ERK1/2 and JNK1 phosphorylation in AAB rats. [5] |
| Kinase Assay | Inhibitory activities of ZLLa1 and ZLLLal against m-calpain and 20S proteasome were measured by previously described methods.For the m-calpain inhibitory assay,the 0.5 ml reaction mixture contained 0.24% alkali-denatured casein,28 mM 2-mercaptoethanol,0.94 unit of m-calpain,ZLLal or ZLLLal,6 mM CaCl2,and 0.1M Tris-HC1 (pH 7.5).The reaction was started by the addition of m-calpain solution and stopped by the addition of 0.5 ml of 10% trichloroacetic acid after incubation at 30℃ for 15 min.After centrifugation at 1,300×g for 10 min,the absorbance of the supernatant at 280 nm was measured.The reaction mixture for the 20S proteasome inhibitory assay contained 0.1 M Tris-acetate,pH 7.0,20S proteasome,ZLLa1 or ZLLLal,and 25 μM substrate dissolved in dimethyl sulfoxide in a final volume of 1 ml.After incubation at 37℃ for 15 min,the reaction was stopped by the addition of 0.1 ml of 10% SDS and 0.9 ml of 0.1 M Tris-acetate,pH 9.0.The fluorescence of the reaction products was measured.To determine the IC50s against m-calpain and 20S proteasome,various concentrations of the synthetic peptide aldehydes were included in the assay mixture [1]. |
| Cell Research | The effect of MG132 on HeLa cell growth was determined by trypan blue exclusion cell counting or measuring MTT dye absorbance of living cells as previously described. In brief, cells (5x10^5 cells per well) were seeded in 24-well plates for cell counting, and cells (5x10^4 cells per well) were seeded in 96-well microtiter plates for the MTT assay. After exposure to indicated amounts of MG132 for 24 h, cells in 24-well plates or 96-well plates were collected with trypsin digestion for trypan blue exclusion cell counting or were used for the MTT assay. Twenty microliters of MTT solution (2 mg/ml in PBS) was added to each well of 96-well plates. The plates were again incubated for 4 h at 37?C. MTT solution in the medium was aspirated off and 200 μl of DMSO was added to each well to solubilize the formazan crystals formed in viable cells. Optical density was measured at 570 nm using a microplate reader. Each plate contained multiple wells at a given experimental condition and multiple control wells. This procedure was replicated for 2-4 plates per condition [3]. |
| Animal Research | Male Sprague–Dawley rats (8 weeks old, 180 – 230 g) were used to establish a pressure-overload model as described previously. All animals were separated into four groups (10 rats per group): (i) vehicle-treated sham group; (ii) MG132-treated sham group; (iii) vehicle-treated abdominal aortic banding (AAB) group; and (iv) MG132-treated AAB group. Under intraperitoneal pentobarbital (50 mg/kg) anesthesia, AAB was created using a 5-0 suture tied twice around the abdominal aorta in which. a 21-gauge needle was inserted. The needle was then retracted yielding a 70 – 80% constriction with an outer aortic diameter of 0.8 mm. In the sham surgery rats, the same surgery was performed as described above except the aorta was constricted. At Day 3 after the surgery, MG132-treated rats were intraperitoneally injected with 0.1 mg/kg/day of MG132 for 8 weeks. All control animals were injected with a corresponding volume of vehicle only (0.1% DMSO) [4]. Sixteen-week-old male CD1 mice were used for all our experiments. Thirty minutes before the immobilization procedure, 0.1 mg/kg of buprenorphine was administrated IP. The mice were then anesthetized using isoflurane. The right hindlimb was immobilized as previously described. Briefly, the hindlimb was immobilized 7 days by stapling the foot exploiting normal dorso-tibial flexion using an Autosuture Royal 35W skin stapler. One tine was inserted close to the toe at the plantar portion of the foot while the other was inserted in the distal portion of the gastrocnemius. The other hindlimb was used as a control. During the immobilization period, the mice were injected subcutaneously with MG132 (7.5 mg/kg/dose) or vehicle (DMSO) twice daily. DMSO containing or not MG132 was diluted in sterile pure corn oil (1:100, injected volume 150 μL). After 7 days, the tibialis anterior (TA) muscles of immobilized and non-i |
| Alias | Z-LLL-al, Z-Leu-Leu-Leu-CHO |
| Molecular Weight | 475.62 |
| Formula | C26H41N3O5 |
| Cas No. | 133407-82-6 |
| Smiles | CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |
| Relative Density. | 1.073 g/cm3 |
| Storage | store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||
| Solubility Information | H2O: Insoluble Ethanol: 47.5 mg/mL (99.87 mM), Sonication is recommended. 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 9 mg/mL (18.92 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. DMSO: 45 mg/mL (94.61 mM), Sonication is recommended. | |||||||||||||||
Solution Preparation Table | ||||||||||||||||
DMSO/Ethanol
| ||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.