Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

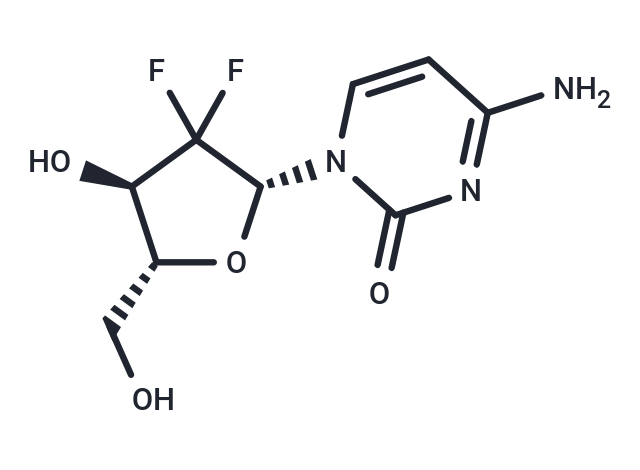
Gemcitabine (LY188011) is a synthetic cytosine nucleoside derivative and an inhibitor of DNA synthesis. Gemcitabine has antitumor and antimetabolic activities. Gemcitabine induces autophagy and apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 50 mg | $34 | In Stock | |
| 100 mg | $50 | In Stock | |
| 200 mg | $58 | In Stock | |
| 500 mg | $85 | In Stock | |
| 1 g | $98 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $50 | In Stock |
| Description | Gemcitabine (LY188011) is a synthetic cytosine nucleoside derivative and an inhibitor of DNA synthesis. Gemcitabine has antitumor and antimetabolic activities. Gemcitabine induces autophagy and apoptosis. |
| Targets&IC50 | BxPC-3 cells:18nM, Capan-2 cells:12 nM, MIAPaCa2:40nM |
| In vitro | METHODS: PDAC-derived paired primary cancer cells (PCCs) PCC-1, PCC-2, PCC-5, PCC-6, and PDAC cells BxPC-3, Mia PaCa-2, and Panc-1 were treated with Gemcitabine (0.001-1000 µM) for 48 h, and the cells were assayed for cell growth inhibition using MTT. RESULTS: Gemcitabine dose-dependently inhibited the growth of PCC-1, PCC-2, PCC-5, PCC-6, BxPC-3, Mia PaCa-2, and Panc-1 cells with IC50 of 1.2/0.3/1.2/4.3/4.2/7.9/10.5 µM, respectively.[1] METHODS: Human pancreatic cancer cells PK-1 were treated with Gemcitabine (30 nM) for 24-48 h. The cell cycle was examined by Flow Cytometry. RESULTS: Gemcitabine induced an increase in the percentage of PK-1 cells in the G0/G1 phase and a decrease in the percentage of S-phase and G2/M cells, and Gemcitabine induced S-phase cell cycle arrest in PK-1 cells. [2] METHODS: Human lung cancer cells SPC-A1 and A549 were transfected with GFP-labeled LC3, incubated with Gemcitabine (5 μM) for 24 h, and then LC3 expression was detected by confocal laser scanning microscopy. RESULTS: The accumulation of LC3-II is a marker of autophagy. Gemcitabine significantly increased the GFP-LC3 spots in the tumor cells, indicating an increase in the level of autophagy. [3] |
| In vivo | METHODS: To detect anti-tumor activity in vivo, Gemcitabine (20 mg/kg) was intraperitoneally injected into BALB/cAJcl-nu/nu mice bearing human high-grade meningioma tumor HKBMM twice a week for four weeks. RESULTS: Gemcitabine treatment not only inhibited tumorigenesis but also tumor growth. Gemcitabine blocked the cell cycle progression and promoted apoptosis in tumor cells in vivo. Gemcitabine exerted potent anti-tumor activity against high-grade meningiomas through cytostatic and cytotoxic mechanisms. [4] METHODS: To assay antitumor activity in vivo, Gemcitabine (50 mg/kg/twice weekly/peritoneal injection) and DMAPT (40 mg/kg/day/gavage) were administered to LSL-KrasG12D/+; LSL-Trp53R172H; and Pdx-1-Cre mutant mice bearing pancreatic cancer tumors. RESULTS: Gemcitabine or the DMAPT/Gemcitabine combination significantly increased median survival (254.5 or 255 versus 217.5 days) and decreased the incidence and diversity of pancreatic adenocarcinomas. Gemcitabine treatment increased plasma levels of IL-1α, IL-1β, and IL-17 in mice. While DMAPT/Gemcitabine decreased the levels of IL-12p40, MCP-1, MIP-1β, eotaxin and TNF-α, all target genes of κB. [5] |
| Cell Research | The cytotoxic effect of gemcitabine was evaluated with the MTT assay. SPC-A1 or A549 cells were treated with gemcitabine (0.05–500 lM) for 24 h. Then, 10 ll of MTT (5 mg/ml in PBS) was added to each well and incubated for 4 h at 37 C. Then, the formazan crystals were solubilized with 200 ll DMSO. The absorbance at 570 nm was measured using an automatic multiwell spectrophotometer. The experiment was repeated four times for each group [3]. |
| Animal Research | At 1 month of age, LSL-Kras G12D/+; LSL-Trp53 R172H; Pdx-1-Cre mice are randomized into treatment groups (placebo, DMAPT, Gemcitabine, DMAPT/Gemcitabine). Placebo (vehicle=hydroxylpropyl methylcellulose, 0.2% Tween 80 [HPMT]) and DMAPT (40 mg/kg body weight in HPMT) are administered by oral gastric lavage once daily. Gemcitabine (50 mg/kg body weight in PBS) is administered by intraperitoneal injection twice weekly. Mouse weight is monitored weekly. Treatment is continued until mice show signs of lethargy, abdominal distension or weight loss at which time they are sacrificed. Successful excision-recombination events are confirmed in the pancreata of mice by detecting the presence of a single LoxP site [5]. |
| Alias | NSC 613327, LY188011 |
| Molecular Weight | 263.2 |
| Formula | C9H11F2N3O4 |
| Cas No. | 95058-81-4 |
| Smiles | NC1=NC(=O)N(C=C1)[C@@H]1O[C@H](CO)[C@@H](O)C1(F)F |
| Relative Density. | 1.84 g/cm3 (Predicted) |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | H2O: 6.25 mg/mL (23.75 mM), Sonication is recommended. DMSO: 125 mg/mL (474.92 mM), Sonication is recommended. Ethanol: 12.5 mg/mL (47.49 mM), Sonication is recommended. 5% DMSO+95% Saline: 0.75 mg/mL (2.85 mM), In vivo: Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
H2O/Ethanol/DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.