- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Shopping Cart
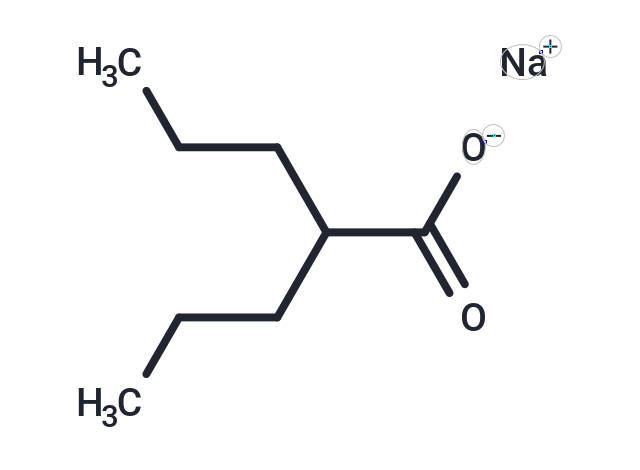
Valproic acid sodium salt
Catalog No. T1602Cas No. 1069-66-5
Alias Sodium Valproate
Valproic acid sodium salt (Sodium Valproate) is the sodium salt form of valproic acid with anti-epileptic activity. Valproic acid sodium salt is converted into its active form, valproate ion, in blood. Although the mechanism of action remains to be elucidated, Valproic acid sodium salt increases concentrations of gamma-aminobutyric acid (GABA) in the brain, probably due to inhibition of the enzymes responsible for the catabolism of GABA. This potentiates the synaptic actions of GABA. Valproic acid sodium salt may also affect potassium channels, thereby creating a direct membrane-stabilizing effect.
Valproic acid sodium salt
Catalog No. T1602Alias Sodium ValproateCas No. 1069-66-5
Valproic acid sodium salt (Sodium Valproate) is the sodium salt form of valproic acid with anti-epileptic activity. Valproic acid sodium salt is converted into its active form, valproate ion, in blood. Although the mechanism of action remains to be elucidated, Valproic acid sodium salt increases concentrations of gamma-aminobutyric acid (GABA) in the brain, probably due to inhibition of the enzymes responsible for the catabolism of GABA. This potentiates the synaptic actions of GABA. Valproic acid sodium salt may also affect potassium channels, thereby creating a direct membrane-stabilizing effect.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 500 mg | $42 | In Stock | |
| 1 g | $50 | In Stock | |
| 5 g | $58 | In Stock | |
| 25 g | $66 | In Stock |
Bulk & Custom
Add to Cart
Questions
View MoreSelect Batch
Purity:99.78%
Contact us for more batch information
All TargetMol products are for research purposes only and cannot be used for human consumption. We do not provide products or services to individuals. Please comply with the intended use and do not use TargetMol products for any other purpose.Product Introduction
Bioactivity
Chemical Properties
| Description | Valproic acid sodium salt (Sodium Valproate) is the sodium salt form of valproic acid with anti-epileptic activity. Valproic acid sodium salt is converted into its active form, valproate ion, in blood. Although the mechanism of action remains to be elucidated, Valproic acid sodium salt increases concentrations of gamma-aminobutyric acid (GABA) in the brain, probably due to inhibition of the enzymes responsible for the catabolism of GABA. This potentiates the synaptic actions of GABA. Valproic acid sodium salt may also affect potassium channels, thereby creating a direct membrane-stabilizing effect. |
| Targets&IC50 | HDAC1:0.4 mM |
| In vitro | In the MT-450 rat mammary carcinoma model, Valproic acid exhibits a delayed effect on the growth of primary tumors. |
| In vivo | In cultured cells, Valproic acid induces histone deacetylation similarly to the histone deacetylase inhibitor Trichostatin A, and like Trichostatin A, activates the transcription of various exogenous and endogenous promoters. However, in the embryos of vertebrates, both Valproic acid and Trichostatin A exhibit teratogenic effects without activating transcription. Valproic acid directly inhibits histone deacetylases through distinct pathways, with an IC50 of 0.4 mM for HDAC1. It inhibits cell proliferation or survival in F9 and P19 teratocarcinoma cells, as indicated by a decrease in [3H]thymidine incorporation, and promotes peroxisome proliferation in the liver of rodents. At a concentration of 1 mM, Valproic acid inhibits the release of Gal4 fused with N-COR, TR, or PPARδ in cells expressing the DNA binding domain of the glucocorticoid receptor and the ligand-binding domain of PPARδ, along with a GR-controlled reporter gene fusion. Valproic acid reduces the accumulation of acetylated histones and inhibits HDAC activity. Furthermore, Valproic acid induces specific types of differentiation, characterized by decreased proliferation, morphological changes, accumulation of the transcription factor AP-2, and expression of marker genes, where AP-2 serves as a potential marker for neuronal or neuroepithelial-like differentiation in F9 teratocarcinoma cells. |
| Kinase Assay | The activity of caspase-3, -8 and -9 is assessed using the caspase-3, -8 and -9 colorimetric assay kits, respectively. In brief, 1×106?cells in a 60-mm culture dish are incubated with 10 mM Valproic acid for 24 h. The cells are then washed in PBS and suspended in 5 volumes of lysis buffer provided with the kit. Protein concentrations are determined using the Bradford method. Supernatants containing 50 μg total protein are used to determine caspase-3, -8 and -9 activities. The supernatants are added to each well in 96-well microtiter plates with DEVD-pNA, IETD-pNA or LEHD-pNA as caspase-3, -8 and -9 substrates and the plates are incubated at 37°C for 1 h. The optical density of each well is measured at 405 nm using a microplate reader. The activity of caspase-3, -8 and -9 is expressed in arbitrary absorbance units. |
| Cell Research | Valproic acid is dissolved in DMSO. In brief, 5×105?cells are seeded in 96-well microtiter plates for MTT assays. After exposure to the designated doses of Valproic acid for the indicated times, MTT solution [20 mL: 2 mg/mL in phosphate-buffered saline (PBS)] is added to each well of the 96-well plates. The plates are additionally incubated for 3 h at 37°C. Medium is withdrawn from the plates by pipetting and 200 mL DMSO is added to each well to solubilize the formazan crystals. The optical density is measured at 570 nm using a microplate reader. |
| Alias | Sodium Valproate |
| Molecular Weight | 166.2 |
| Formula | C8H15NaO2 |
| Cas No. | 1069-66-5 |
| Smiles | [Na+].CCCC(CCC)C([O-])=O |
| Relative Density. | 1.0803 g/cm3 |
Storage & Solubility Information
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | DMSO: 50 mg/mL (300.84 mM), Sonication is recommended. H2O: 16.6 mg/mL (99.88 mM), Sonication is recommended. | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
H2O/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||
Calculator
In Vivo Formulation Calculator (Clear solution)
Please enter your animal experiment information in the following box and click Calculate to obtain the mother liquor preparation method and in vivo formula preparation method:
Mother liquor preparation method: 2 mg of drug dissolved in 50 μL DMSO (mother liquor concentration of 40 mg/mL), if you need to configure a concentration that exceeds the solubility of the product, please contact us first.
(mother liquor concentration of 40 mg/mL), if you need to configure a concentration that exceeds the solubility of the product, please contact us first.
Preparation method for in vivo formula: Take 50 μL DMSO main solution, add 300 μLPEG300
main solution, add 300 μLPEG300 mix well and clarify, then add 50 more μL Tween 80, mix well and clarify, then add 600 more μLddH2O
mix well and clarify, then add 50 more μL Tween 80, mix well and clarify, then add 600 more μLddH2O mix well and clarify
mix well and clarify
For Reference Only. Please develop an appropriate dissolution method based on your laboratory animals and route of administration.
Dose Conversion
You can also refer to dose conversion for different animals. More Dose Conversion
Tech Support
Please see Inhibitor Handling Instructions for more frequently ask questions. Topics include: how to prepare stock solutions, how to store products, and cautions on cell-based assays & animal experiments, etc
Keywords
Valproic acid sodium saltValproic acid sodiumValproic acidsmall cell lung cancerSCLCproteasomalNotch1NotchMitophagyMitochondrial AutophagymigraineInhibitorinhibitHuman immunodeficiency virusHIVProteaseHIV ProteaseHIVHistone deacetylaseshepatic fat accumulationheadachesHDAC9HDAC2HDACGammasecretaseGABAReceptorGABARGABA ReceptorepilepsyEndogenous Metabolitedegradationbipolar disorderAutophagyApoptosisanticonvulsantanticancer
Related Tags: buy Valproic acid sodium salt | purchase Valproic acid sodium salt | Valproic acid sodium salt cost | order Valproic acid sodium salt | Valproic acid sodium salt chemical structure | Valproic acid sodium salt in vivo | Valproic acid sodium salt in vitro | Valproic acid sodium salt formula | Valproic acid sodium salt molecular weight

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.



