Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

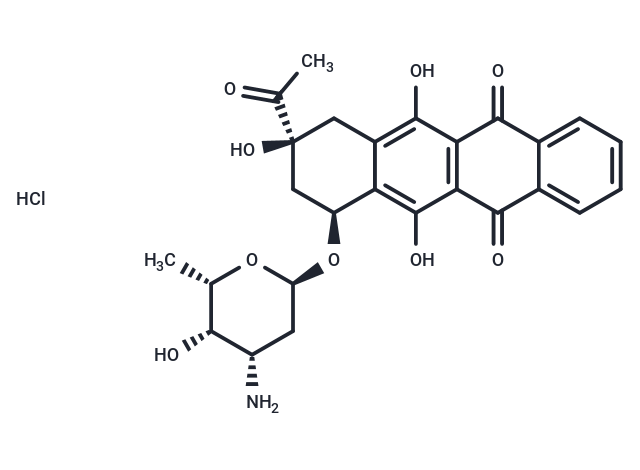
Idarubicin hydrochloride (Zavedos), a hydrochloride salt form of Idarubicin, is a DNA topoisomerase II (topo II) inhibitor for MCF-7 cells ( IC50: 3.3 ng/mL).
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $55 | In Stock | |
| 10 mg | $97 | In Stock | |
| 25 mg | $163 | In Stock | |
| 50 mg | Inquiry | In Stock | |
| 1 mL x 10 mM (in DMSO) | $67 | In Stock |
| Description | Idarubicin hydrochloride (Zavedos), a hydrochloride salt form of Idarubicin, is a DNA topoisomerase II (topo II) inhibitor for MCF-7 cells ( IC50: 3.3 ng/mL). |
| Targets&IC50 | Topo II (MCF-7 cells):3.3 ng/mL |
| In vitro | Idarubicin has significant cytotoxic activity against multicellular spheroids, comparable to the antiproliferative effects on monolayer cells. [1] Idarubicin inhibits CYP450 2D6.[2] Idarubicin is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively. Idarubicin is able to overcome P-glycoprotein-mediated multidrug resistance. [3] Idarubicin inhibits PMN superoxide radical formation. [4] Idarubicin could be coupled to the monoclonal antibodies (anti-Ly-2.1, anti-L3T4, or anti-Thy-1) with retention of protein solubility and antibody activity. [5] Idarubicin inhibits the proliferation of NALM-6 cells with an IC50 of 12 nM. [6] |
| In vivo | Reduction of Idarubicin is dependent upon ketone reductases, and proceeds more stereoselectively than that of most ketones giving rise to the (13S)-epimer almost exclusively. The high stereospecificity in Idarubicin reduction might result from chiral induction due to the presence of asymmetric centres near to the carbonyl group in Idarubicin. [7] |
| Kinase Assay | CYP450 metabolism experiments: Evaluation of Idarubicin metabolism by the CYP450 isoenzymes 3A4, 2D6, 2C8, 2C9, and 1A2 is completed using isolated human CYP450 proteins for each isoform. The high throughput P450 inhibition testing method is utilized for these evaluations. The metabolism experiments are designed to investigate the following properties of each drug: (1) if Idarubicin is a substrate of the CYP450 3A4, 2C8, 2C9, 1A2 or 2D6 isoenzymes; (2) if metabolism is affected by known inhibitors of each isoenzyme; (3) if Idarubicin is inhibitors of CYP450 isoenzymes; and (4) if caspofungin or itraconazole inhibit the CYP450 metabolism of Idarubicin. Dibenzylfluorescein (DBF) (CYP3A4, CYP2C8, CYP2C9), 3-cyano-7-ethoxycoumarin (Cyp1A2), and 7-methoxy-4-(aminomethyl)-coumarin (MAMC) (CYP2D6) are the known substrates utilized as controls to confirm the respective isoenzyme activity and evaluate the effects of Idarubicin on the isoenzyme activity. In addition, ketoconazole, quercetin, suflaphenazole, furafylline, and quinidine are utilized as control CYP450 inhibitors for 3A4, 2C8, 2C9, 1A2 or 2D6 isoenzymes, respectively. The substrate, inhibitor plus Idarubicin as indicated are added to each protein sample are incubated for 20 minutes- 60 minutes, as recommend by manufacturer, at 37oC. Reactions are stopped with an organic solvent solution and then samples are analyzed by fluorescence plate reader as appropriate. For each experiment, control samples with a known amount of substrate and synthesized metabolite, in the absence of the isoenzyme, are prepared for qualitative comparisons. All experiments are performed in triplicate. |
| Cell Research | The anti-proliferative activity of the Idarubicin in the conjugate is compared to that of free drug by measuring the inhibition of [3H]thymidine uptake. Briefly, NALM-6 cells (1.5 × 106/mL) are added to a flat-bottomed microtitre plate (100 μL/well) and incubated for 1 hours at 37ºC. Free Idarubicin and Idarubicin-mAb conjugates are sterilised by filtration and diluted in sterile PBS; various concentrations are added to the wells (100 μL/well) in duplicate and the plates are incubated at 37ºC, 7% CO2 for 24 hours. Following incubation, 50 μL medium containing 1 μCi [3H]thymidine is added to each well and the plates are incubated for a further 4 hours. Cells are harvested onto glass-fibre filter-paper, dried and counted in a scintillation counter. Specificity studies are performed using the same technique where the ability of Idarubicin-anti-CD19 conjugates to kill CD19 + cells is compared to the cytotoxicity of irrelevant Idarubicin-JGT conjugates. NALM-6 cells (1.5× 106/mL, 300 μL tube) are incubated for 30 rain on ice with various concentrations of Idarubicin-anti-CD 19 or Idarubicin-JGT conjugates. Following three washes in ice-cold RPMI-1640 medium (4 mL/wash), the cells are resuspended in fresh medium and transferred to 96-well plates (100 μL/well). Each tube is set up in duplicate and two wells are plated out per tube (a total of 4 wells per drug concentration). Cells are pulsed with [3H]thymidine 24 hours later and harvested. (Only for Reference) |
| Alias | Zavedos, Idarubicin HCl, Idamycin, 4-Demethoxydaunorubicin hydrochloride, 4-demethoxydaunorubicin (NSC256439, 4-DMDR) HCl |
| Molecular Weight | 533.95 |
| Formula | C26H27NO9·HCl |
| Cas No. | 57852-57-0 |
| Smiles | Cl.C[C@@H]1O[C@H](C[C@H](N)[C@@H]1O)O[C@H]1C[C@@](O)(Cc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c12)C(C)=O |
| Relative Density. | no data available |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||||||||||||||||||||||
| Solubility Information | H2O: 5 mg/mL (9.36 mM), Sonication is recommended. DMSO: 93 mg/mL (174.17 mM), Sonication is recommended. Ethanol: < 1 mg/mL (insoluble or slightly soluble) | ||||||||||||||||||||||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||||||||||||||||||||||
H2O/DMSO
DMSO
| |||||||||||||||||||||||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.