Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Alisertib (MLN 8237) is a specific Aurora A inhibitor (IC50: 1.2 nM). The selectivity of Alisertib(MLN 8237) is >200-fold higher for Aurora A than Aurora B.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $50 | In Stock | |
| 10 mg | $88 | In Stock | |
| 25 mg | $157 | In Stock | |
| 50 mg | $238 | In Stock | |
| 100 mg | $372 | In Stock | |
| 200 mg | $478 | In Stock | |
| 500 mg | $783 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $57 | In Stock |
| Description | Alisertib (MLN 8237) is a specific Aurora A inhibitor (IC50: 1.2 nM). The selectivity of Alisertib(MLN 8237) is >200-fold higher for Aurora A than Aurora B. |
| Targets&IC50 | Aurora A:1.2 nM (cell free) |
| In vitro | Treatment of cultured MM cells with Alisertib (MLN8237) results in mitotic spindle abnormalities, mitotic accumulation, as well as inhibition of cell proliferation through apoptosis and senescence. In addition, MLN8237 up-regulates p53 and tumor suppressor genes p21 and p27. Combining MLN8237 with dexamethasone, doxorubicin, or bortezomib induces synergistic/additive anti-MM activity in vitro [1]. Alisertib inhibited AAK over ABK with a selectivity of more than 200-fold in cells. Alisertib inhibited proliferation of human tumor cell lines in vitro [2]. A T217D/W277E double mutant exhibits superior levels of resistance to MLN8237, with the I50 value increasing approximately 20-fold from 30 to 650 nM in the presence of TPX2 [3]. |
| In vivo | Tumor burden was significantly reduced and overall survival was significantly increased in animals treated with 30 mg/kg MLN8237 for 21 days. Induction of apoptosis and cell death by MLN8237 were confirmed in tumor cells excised from treated animals by TdT-mediated dUTP nick end labeling assay [1]. Alisertib produced a dose-dependent decrease in bipolar and aligned chromosomes in the HCT-116 xenograft model. Alisertib produced tumor growth inhibition in solid tumor xenograft models and regressions in in vivo lymphoma models. In addition, a dose of alisertib that caused tumor stasis, as measured by volume, resulted in a decrease in FLT uptake [2]. Nude mice bearing human tumor xenografts treated with a single oral dose of alisertib (20 mg/kg) displayed a phenotype consistent with inhibition of Aurora A [4]. |
| Kinase Assay | Recombinant murine Aurora A and Aurora B protein were expressed in Sf9 cells and purified with GST affinity chromatography. The peptide substrate for Aurora A was conjugated with biotin (Biotin-GLRRASLG). Aurora A kinase (5 nM) was assayed in 50 mM Hepes (pH 7.5)/10 mM MgCl2/5 mM DTT/0.05% Tween 20/2 μM peptide substrate/3.3 μCi/ml [γ-33P]ATP at 2 μM by using Image FlashPlates. Aurora B kinase (2 nM) was assayed with 10 μM biotinylated peptide Biotin-TKQTARKSTGGKAPR in 50 mM Tricine (pH 8.0)/2.5 mM MgCl2/5 mM DTT/10% glycerol/2% BSA/40 μCi/ml [γ-33P]ATP at 250 μM. The conditions for all other in vitro kinase assays are available upon request. MLN8054 was run in a 226 kinase screen at a 1 μM compound concentration with an ATP concentration of 10 μM for all assays [2]. |
| Cell Research | HCT-116 colorectal carcinoma cells were plated on 6-well dishes (2 × 10^5 per well) and propagated in McCoy's 5A media supplemented with 10% FBS. After 18 hours, alisertib at a final concentration of 0.050, 0.250, or 1.000 μmol/L was added, and the cells were grown for an additional 24 hours. Cells treated with dimethyl sulfoxide (DMSO; 0.2%) served as the untreated vehicle control. The cells were harvested with trypsin EDTA 1×, washed once with PBS, fixed in 70% ethanol, and stored at 4°C for 1 hour. The cells were resuspended in propidium iodide (1:40) and RNAse A (1:5,000) in PBS for 30 minutes at 4°C. Cell-cycle distributions were determined by measuring DNA content using flow cytometry, and samples were analyzed using Winlist 5.0 software [2]. |
| Animal Research | Mice were irradiated (200 cGy), and then 5 × 106 MM1.S cells were inoculated subcutaneously in the right flank. When tumor growth was measurable (~ 2 weeks after the injection), mice were assigned into 4 groups (10 mice each) receiving vehicle orally (100 μL of 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate) or MLN8237 (7.5 mg/kg, 15 mg/kg, and 30 mg/kg in a final formulation in 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate) for 21 consecutive days. The maximal tolerated dose of MLN8237 in most mouse strains (continuous dosing for 21 days) is approximately 20 mg/kg twice a day (40 mg/kg per day). Tumor volumes were measured by a Vernier caliper every alternate day and calculated using the following formula: length × width2 × 0.5. Tumor growth inhibition (TGI) was calculated using the formula (Δcontrol average volume ? Δtreated average volume) × 100/(Δcontrol average volume). Mice were killed at the end of the treatment, 2 hours after the last treatment, or when tumor reached 2 cm^3; tumors were immediately collected from mice and evaluated for induction of apoptosis and cell death by TdT-mediated dUTP nick end labeling (TUNEL) assay [1]. |
| Alias | MLN 8237 |
| Molecular Weight | 518.92 |
| Formula | C27H20ClFN4O4 |
| Cas No. | 1028486-01-2 |
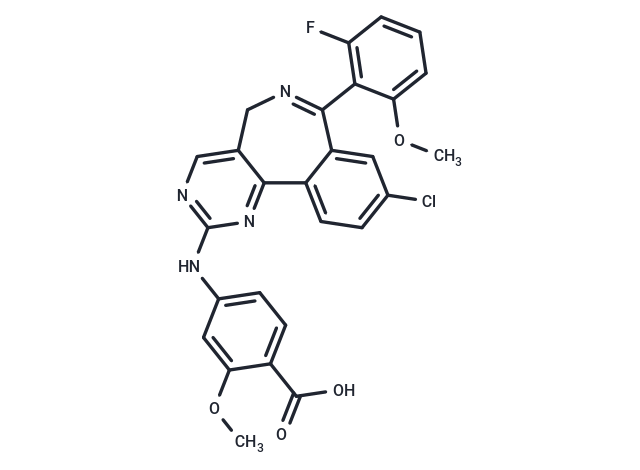
| Smiles | COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |
| Relative Density. | 1.438 g/cm3 |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5 mg/mL (9.64 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. DMSO: 50 mg/mL (96.35 mM), Sonication is recommended. H2O: < 1 mg/mL (insoluble or slightly soluble) Ethanol: < 1 mg/mL (insoluble or slightly soluble) | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.