Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Sorafenib (Bay 43-9006) is a multikinase inhibitor that targets Raf-1, B-Raf, VEGFR2, VEGFR3, VEGFR4, PDGFRβ, FLT3, c-Kit, and others (IC50=6/22/90/15/20/20/57/58 nM) with oral activity. It exhibits antitumor properties and can induce autophagy, apoptosis, and agonistic iron death.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 50 mg | $34 | In Stock | |
| 100 mg | $48 | In Stock | |
| 500 mg | $63 | In Stock | |
| 1 g | $91 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $30 | In Stock |
| Description | Sorafenib (Bay 43-9006) is a multikinase inhibitor that targets Raf-1, B-Raf, VEGFR2, VEGFR3, VEGFR4, PDGFRβ, FLT3, c-Kit, and others (IC50=6/22/90/15/20/20/57/58 nM) with oral activity. It exhibits antitumor properties and can induce autophagy, apoptosis, and agonistic iron death. |
| Targets&IC50 | c-Kit:68 nM (cell free), PDGFRβ:57 nM (cell free), B-Raf V599E:38 nM (cell free), Raf-1:6 nM (cell free0, B-Raf:22 nM (cell free), VEGFR3:20 nM (cell free) |
| In vitro | METHODS: Human hepatocellular carcinoma cells HepG2 and HuH-7 were treated with Sorafenib (2-20 µmol/L) for 48 h, and cell growth inhibition was detected using MTT method.
RESULTS: Sorafenib dose-dependently inhibited the growth of HepG2 and HuH-7 cells with IC50 of approximately 6 µmol/L.[1] METHODS: Human acute promyelocytic leukemia cells NB4 were treated with Sorafenib (1.5-12 µM) for 24-48 h. Apoptosis was detected using Flow Cytometry. RESULTS: Sorafenib dose-dependent apoptosis of NB4 cells, with a significant increase in the proportion of both early and late apoptotic cells. [2] METHODS: Rat hepatobiliary cholangiocarcinoma cells LCC-2 were treated with Sorafenib (2.5-5 μM) for 12 h. Mitochondrial membrane potential was measured using JC-1 dye. RESULTS: Sorafenib depolarized the isolated mitochondria. [3] |
| In vivo | METHODS: To assay antitumor activity in vivo, Sorafenib (7.5-60 mg/kg) was orally administered once daily for two to four days to NCr-nu/nu mice harboring human tumors MDA-MB-231, Colo-205, HT-29, DLD-1, NCI-H460, and A549.
RESULTS: Sorafenib showed broad oral antitumor efficacy in various human tumor xenograft models. [4] METHODS: To assay antitumor activity in vivo, Sorafenib (30 mg/kg/five times per week) and everolimus (10 mg/kg/three times per week) were administered by gavage to PTEN-mutant mice bearing CRPC, a tumor of desmoplasia-resistant prostate cancer, once a day for four weeks. RESULTS: Sorafenib administration increased the expression of androgen receptor p-GSK3β and p-ERK1/2 in CRPC, and the combination of Sorafenib and everolimus overcame treatment escape in CRPC tumors treated with Sorafenib alone. [5] |
| Kinase Assay | Recombinant baculoviruses expressing Raf-1 (residues 305–648) and B-Raf (residues 409–765) are purified as fusion proteins. Full-length human MEK-1 is generated by PCR and purified as a fusion protein from Escherichia coli lysates. Sorafenib tosylate is added to a mixture of Raf-1 (80 ng), or B-Raf (80 ng) with MEK-1 (1 μg) in assay buffer [20 mM Tris (pH 8.2), 100 mM NaCl, 5 mM MgCl2, and 0.15% β-mercaptoethanol] at a final concentration of 1% DMSO. The Raf kinase assay (final volume of 50 μL) is initiated by adding 25 μL of 10 μM γ[33P]ATP (400 Ci/mol) and incubated at 32 °C for 25 minutes. Phosphorylated MEK-1 is harvested by filtration onto a phosphocellulose mat, and 1% phosphoric acid is used to wash away unbound radioactivity. After drying by microwave heating, a β-plate counter is used to quantify filter-bound radioactivity. Human VEGFR2 (KDR) kinase domain is expressed and purified from Sf9 lysates. Time-resolved fluorescence energy transfer assays for VEGFR2 are performed in 96-well opaque plates in the time-resolved fluorescence energy transfer format. Final reaction conditions are as follows: 1 to 10 μM ATP, 25 nM poly GT-biotin, 2 nM Europium-labeled phospho (p)-Tyr antibody (PY20), 10 nM APC, 1 to 7 nM cytoplasmic kinase domain in final concentrations of 1% DMSO, 50 mM HEPES (pH 7.5), 10 mM MgCl2, 0.1 mM EDTA, 0.015% Brij-35, 0.1 mg/mL BSA, and 0.1% β-mercaptoethanol. Reaction volumes are 100 μL and are initiated by the addition of enzyme. Plates are read at both 615 and 665 nM on a Perkin-Elmer VictorV Multilabel counter at ~1.5 to 2.0 hours after reaction initiation. Signal is calculated as a ratio: (665 nm/615 nM) × 10,000 for each well. For IC50 generation, Sorafenib tosylate is added before the enzyme initiation. A 50-fold stock plate is made with Sorafenib tosylate serially diluted 1:3 in a 50% DMSO/50% distilled water solution. Final Sorafenib tosylate concentrations range from 10 μM to 4.56 nM in 1% DMSO. |
| Cell Research | Tumor cell lines were plated at 2 × 105 cells per well in 12-well tissue culture plates in DMEM growth media (10% heat-inactivated FCS) overnight. Cells were washed once with serum-free media and incubated in DMEM supplemented with 0.1% fatty acid-free BSA containing various concentrations of BAY 43-9006 in 0.1% DMSO for 120 minutes to measure changes in basal pMEK 1/2, pERK 1/2, or pPKB. Cells were washed with cold PBS (PBS containing 0.1 mmol/L vanadate) and lysed in a 1% (v/v) Triton X-100 solution containing protease inhibitors. Lysates were clarified by centrifugation, subjected to SDS-PAGE, transferred to nitrocellulose membranes, blocked in TBS-BSA, and probed with anti-pMEK 1/2 (Ser217/Ser221; 1:1000), anti-MEK 1/2, anti-pERK 1/2 (Thr202/Tyr204; 1:1000), anti-ERK 1/2, anti-pPKB (Ser473; 1:1000), or anti-PKB primary antibodies. Blots were developed with horseradish peroxidase (HRP)-conjugated secondary antibodies and developed with Amersham ECL reagent on Amersham Hyperfilm [1]. |
| Animal Research | Female NCr-nu/nu mice (Taconic Farms, Germantown, NY) were used for all studies. Three to five million cells were injected s.c. into the right flank of each mouse. DLD-1 tumors were established and maintained as a serial in vivo passage of s.c. fragments (3 × 3 mm) implanted in the flank using a 12-gauge trocar. A new generation of the passage was initiated every three weeks, and studies were conducted between generations 3 and 12 of this line. Treatment was initiated when tumors in all mice in each experiment ranged in size from 75 to 144 mg for antitumor efficacy studies and from 100 to 250 mg for studies of microvessel density and ERK phosphorylation. All treatment was administered orally once daily for the duration indicated in each experiment. |
| Alias | Bay 43-9006 |
| Molecular Weight | 464.82 |
| Formula | C21H16ClF3N4O3 |
| Cas No. | 284461-73-0 |
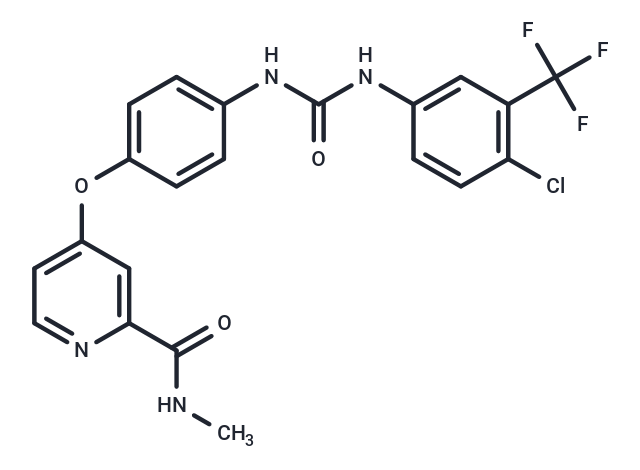
| Smiles | O(C=1C=C(C(NC)=O)N=CC1)C2=CC=C(NC(NC3=CC(C(F)(F)F)=C(Cl)C=C3)=O)C=C2 |
| Relative Density. | 1.455. Temperature:20. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | H2O: < 1 mg/mL (insoluble or slightly soluble) 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 5.9 mg/mL (12.69 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. DMF: 3.33 mg/mL (7.16 mM), Sonication is recommended. DMSO: 55 mg/mL (118.33 mM), Sonication is recommended. Ethanol: < 1 mg/mL (insoluble or slightly soluble) | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.