Shopping Cart
- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

Venetoclax (ABT-199) is a Bcl-2 inhibitor (Ki<0.01 nM) with potent, selective, and orally active properties. Venetoclax has a 3-order-of-magnitude lower affinity for Bcl-xL and Bcl-W (Kis=48/245 nM). Venetoclax induces autophagy and apoptosis.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 5 mg | $48 | In Stock | |
| 10 mg | $68 | In Stock | |
| 50 mg | $89 | In Stock | |
| 100 mg | $116 | In Stock | |
| 500 mg | $382 | In Stock | |
| 1 g | $596 | In Stock | |
| 1 mL x 10 mM (in DMSO) | $66 | In Stock |
| Description | Venetoclax (ABT-199) is a Bcl-2 inhibitor (Ki<0.01 nM) with potent, selective, and orally active properties. Venetoclax has a 3-order-of-magnitude lower affinity for Bcl-xL and Bcl-W (Kis=48/245 nM). Venetoclax induces autophagy and apoptosis. |
| Targets&IC50 | BCL-XL:48 nM (Ki, cell free), BCL2:<0.01 nM (Ki, cell free) |
| In vitro | METHODS: Eleven human T-cell acute lymphoblastic leukemia cells T-ALL were treated with Venetoclax (0-12 μM) for 48 h. Cell viability was measured by Celltiter-Glo Luminescent Cell Viability Assay. RESULTS: The IC50 values of Venetoclax against 11 T-ALL species ranged from 0.2-10 μM. [1] METHODS: Human acute lymphoid leukemia cells RS4;11 were incubated with Venetoclax (0.01-5 μM) for 3.5 h. Caspase-3/7 activity was assessed using the Caspase-GLO kit. RESULTS: Venetoclax induced the activation of caspases, which is one of the characteristics of apoptosis. [2] METHODS: Human primary HCL leukemia cells were treated with Venetoclax (0.1-1 μM) for 24 h. Cell death was detected using Flow Cytometry method. RESULTS: Venetoclax significantly increased cell death in HCL cells in a dose-dependent manner. [3] |
| In vivo | METHODS: To test the antitumor activity in vivo, Venetoclax (100 mg/kg in 60% PG+30% PEG 400+10% ethanol) was orally administered once daily for twenty-one days to C.B-17 SCID-beige mice bearing human diffuse large B-cell lymphoma Toledo. RESULTS: Venetoclax significantly inhibited the growth of Toledo tumor (TGImax=93%, TGD=220%). [2] METHODS: To assay anti-tumor activity in vivo, Venetoclax (50 mg/kg in 10% ethanol+30% PEG 400+60% Phosal 50PG, administered orally once daily) and anti-PD-1 (10 mg/kg in PBS, administered intraperitoneally three times every four days) were administered to C57BL/6 mice harboring mouse colorectal carcinoma tumor MC38 for fourteen days. RESULTS: Venetoclax enhances the antitumor efficacy of immune checkpoint inhibitors (ICIs) and increases PD-1+ T effector memory cells. Venetoclax does not impair human T-cell function in response to antigenic stimulation in vitro, nor does it antagonize anti-PD-1-induced T-cell activation. [4] |
| Kinase Assay | The equilibrium binding experiments of fluorescent peptides to Bcl-xL protein were performed in an Analyst 96-well plate reader under the following conditions: each individual well in a 96-well assay plate contained 5 μl DMSO, 15 nM fluorescent peptide, and increasing concentrations (from 0 to 2.24 μM) of Bcl-xL protein in assay buffer in a final volume of 125 μl. The plate was mixed on a shaker for 1 min and incubated at room temperature for an additional 15 min. The polarization in millipolarization units (mP) was measured at room temperature with an excitation wavelength at 485 nm and an emission wavelength at 530 nm. For assay stability testing, a plate containing a binding experiment was measured at different times over a 24-h period. Between each reading, the plate was covered with parafilm to prevent any solution evaporation. To determine the effect of DMSO on the assay, binding experiments were performed under conditions similar to those described above except that the amount of DMSO was varied from 0 to 4 to 8%. All experimental data were analyzed using Prism 3.0 software and Kd values were generated by fitting the experimental data using a sigmoidal dose-response nonlinear regression model [1]. |
| Cell Research | RS4;11 cells were seeded at 50,000 per well in 96-well plates and treated with compounds diluted in half-log steps starting at 1 μM and ending at 0.00005 μM. All other leukemia and lymphoma cell lines were seeded at 15,000–20,000 cells per well in the appropriate medium and incubated with ABT-199 or navitoclax for 48 h. Effects on proliferation were determined using Cell TiterGlo reagent. EC50 values were determined by nonlinear regression analysis of the concentration-response data. Mouse FL5.12–BCL-2 and FL5.12–BCL-XL cells were propagated and assessed as described previously. Bak?/? Bax?/? double knockout mouse embryonic fibroblasts were seeded into 96-well microtiter plates at 5,000 cells per well in DMEM supplemented with 10% FBS. ABT-199 in the same culture medium was added in half-log dilutions starting at 5 μM. The cells were then incubated at 37 °C (5% CO2) for 48 h, and the effects on proliferation were determined using Cell TiterGlo reagent according to the manufacturer's instructions [1]. |
| Animal Research | Female C.B-17 SCID mice (DoHH2 and Granta-519 xenografts) and female C.B-17 SCID-beige mice (RS4;11 and Toledo xenografts) were inoculated with 1 × 10^6 (DoHH2) or 5 × 10^6 (Granta-519, Toledo and RS4;11) cells subcutaneously in the right flank. The inoculation volume (0.2 ml) comprised a 50:50 mixture of cells in growth media and Matrigel. Electronic calipers were used to measure the length and width of each tumor 2–3 times per week. Tumor volume was estimated by applying the following equation: volume = length × width2/2. When tumors reached approximately 220 mm3, mice were size matched (day 0) into treatment and control groups. All xenograft trials were conducted using ten mice per group, and all mice were ear tagged and monitored individually throughout the studies. ABT-199 was formulated for oral dosing in 60% phosal 50 propylene glycol (PG), 30% polyethylene glycol (PEG) 400 and 10% ethanol, and bendamustine and rituximab were formulated in accordance with the manufacturer's instructions. ABT-199 was delivered approximately 2 h before bendamustine or bendamustine plus rituximab. TGImax was calculated as the greatest treatment response using the following equation: TGImax = (1 ? mean tumor volume of the treated group/mean tumor volume of the vehicle control group) × 100. The TGD (%) was determined as the percentage increase of the median time period for the treatment group to reach an arbitrary tumor volume of 1,000 mm3 relative to the vehicle control group. A complete tumor regression response was the portion of the population with tumors ≤25 mm3 for at least three consecutive measurements [1]. |
| Alias | GDC-0199, ABT-199, ABT199, ABT 199 |
| Molecular Weight | 868.44 |
| Formula | C45H50ClN7O7S |
| Cas No. | 1257044-40-8 |
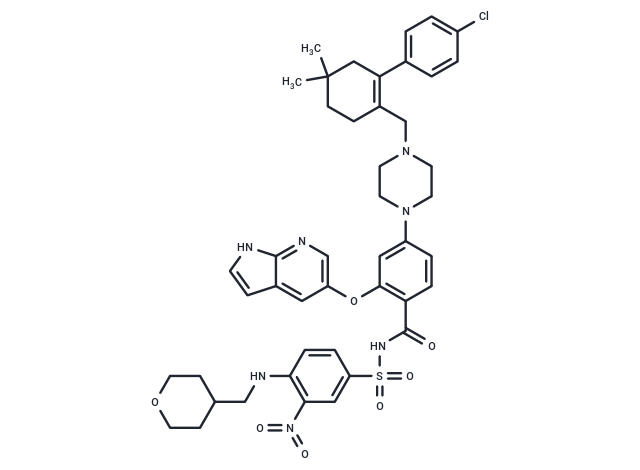
| Smiles | O=C(C1=CC=C(N2CCN(CC2)CC3=C(CC(C)(CC3)C)C4=CC=C(Cl)C=C4)C=C1OC5=CC6=C(NC=C6)N=C5)NS(=O)(C7=CC=C(C([N+]([O-])=O)=C7)NCC8CCOCC8)=O |
| Relative Density. | 1.340 g/cm3 (Predicted) |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | ||||||||||||||||||||
| Solubility Information | H2O: < 1 mg/mL (insoluble or slightly soluble) 10% DMSO+40% PEG300+5% Tween 80+45% Saline: 10 mg/mL (11.51 mM), suspension.In vivo: Please add the solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately. Ethanol: < 1 mg/mL (insoluble or slightly soluble) DMSO: 100 mg/mL (115.15 mM), Sonication is recommended. | ||||||||||||||||||||
Solution Preparation Table | |||||||||||||||||||||
DMSO
| |||||||||||||||||||||

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.