- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
NEWS | 24 May 2024
WIKIMOLE—Osimertinib
By TargetMol
Osimertinib is an orally available, third-generation EGFR inhibitor (EGFR-TKI) widely used for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations.
Background
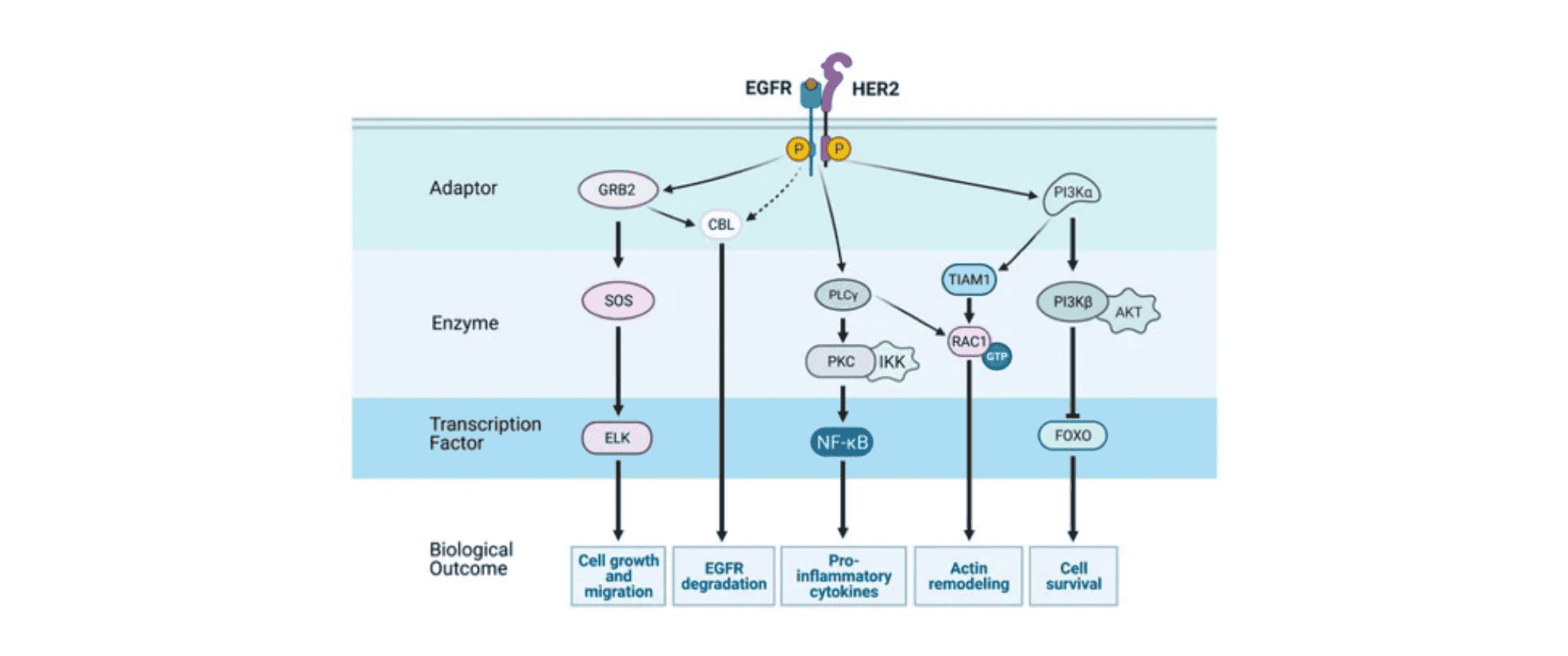
EGFR (Epidermal Growth Factor Receptor) is the receptor for epidermal growth factor (EGF) involved in cell proliferation and signal transduction. It is also known as HER1 or ErbB1. The EGFR signaling network is highly complex and consists of multiple layers. The input layer includes receptors and ligands, while beneath the cell surface lies an intricate system of various enzymes, adaptor proteins, second messengers, and transcription factors. The output layer encompasses various cellular responses. In most cases, the ultimate result of EGFR activation is the stimulation of cell growth. Existing research has shown that activating mutations of the EGFR gene are key oncogenic drivers in non-small cell lung cancer (NSCLC).
Application
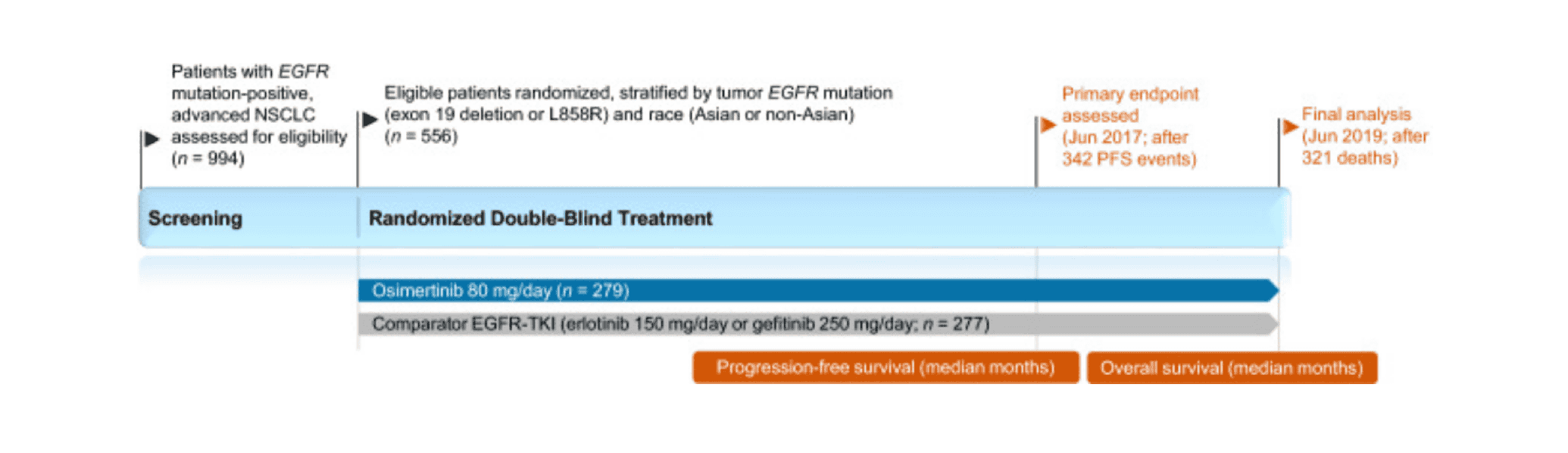
The emergence of EGFR-TKIs in the early 21st century marked a paradigm shift in the management of EGFR mutation-positive NSCLC, heralding the advent of the era of precision medicine. Compared to traditional cytotoxic therapies, EGFR-TKIs have improved clinical outcomes and health-related quality of life for patients with advanced disease. However, acquired resistance to first-generation (e.g., erlotinib, gefitinib) and second-generation (e.g., afatinib, dacomitinib) EGFR-TKIs typically emerges after a median of 9-15 months, with the EGFR T790M mutation appearing to mediate resistance in approximately 50%-60% of patients.
Third-generation EGFR-TKIs were designed to selectively inhibit both the sensitizing and T790M resistance mutations of EGFR tyrosine kinase, while largely sparing wild-type EGFR activity, thereby reducing off-target toxicity compared to earlier EGFR-TKIs. Osimertinib, a third-generation EGFR inhibitor (EGFR-TKI), has been widely approved for the first-line treatment of advanced NSCLC with activating EGFR mutations, and has also shown potential efficacy against other types of EGFR mutation tumors in clinical trials. With a deeper understanding of resistance mechanisms and continuous innovation in therapeutic strategies, osimertinib’s position in lung cancer treatment is expected to further consolidate and expand.
Reference
[1] doi:10.1016/s0959-8049(01)00230-1
[2] doi:10.1007/s11523-021-00839-w
[3] doi: 10.1111/cas.15229.
[4] doi: 10.1038/s41598-024-58499-5.
Other Articles

Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2024 TargetMol Chemicals Inc. All Rights Reserved.











