- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
NEWS | 06 September 2024
WIKIMOLE—Necrostatin-1(Nec-1)
By TargetMol
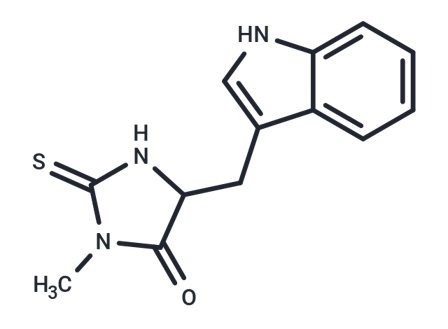
Necrostatin-1 (Nec-1)(Product No. T1847, CAS 4311-88-0) is a necroptosis inhibitor targeting RIPK1 and also an IDO inhibitor. Necrostatin-1 consists of a benzene ring, an aminothiazole ring, and an amide group. These structural features enable it to specifically bind to RIPK1 and inhibit its activity.
Mechanism of Action
Necrosis is a form of programmed cell death that typically occurs when cells suffer severe damage. Unlike classical apoptosis, necrosis is often accompanied by cell membrane rupture and the release of cellular contents, leading to an inflammatory response. Key regulatory molecules of necrosis include RIPK1, RIPK3, and MLKL (Mixed-Lineage Kinase Domain-Like Protein). In the necroptosis signaling pathway, activation of RIPK1 and RIPK3 forms a necrosome complex, which activates MLKL, leading to cell membrane rupture and necrosis[1].
Necrostatin-1 (Nec-1) is a compound used to inhibit programmed necrosis. It binds to RIPK1 and inhibits its kinase activity, preventing RIPK1 from interacting with RIPK3, thereby reducing MLKL activation and cell membrane rupture, interrupting the necroptosis process.
Additionally, Nec-1 reduces the inflammatory response caused by cell necrosis by inhibiting RIPK1. It also regulates tumor immunity by inhibiting indoleamine 2,3-dioxygenase (IDO)[2].
Application
-ResearchonNeurologicalDiseases
1.In the cerebral ischemia-reperfusion injury model, necroptosis is considered one of the key mechanisms of neuronal death. Studies have found that p-RIPK1 (Ser166) increases in the brain after ischemia, activating the RIPK3/MLKL-dependent necrotic signaling pathway, leading to neuronal death. Necrostatin-1 treatment can prevent ischemic neuronal death by inhibiting RIPK1-mediated, RIPK3/MLKL-dependent necrosis in the brains of rats following ischemic stroke[4].
2.In Parkinson's disease (PD) models, Rotenone is used to mimic the PD condition in cells. Rotenone triggers necroptosis by inhibiting mitochondrial complex I and increasing the production of reactive oxygen species (ROS), which activate necroptosis through RIP1. Necrostatin-1 can inhibit the phosphorylation of RIP1, preventing the formation of the RIP1 and RIP3 complex, thereby reducing necroptosis.
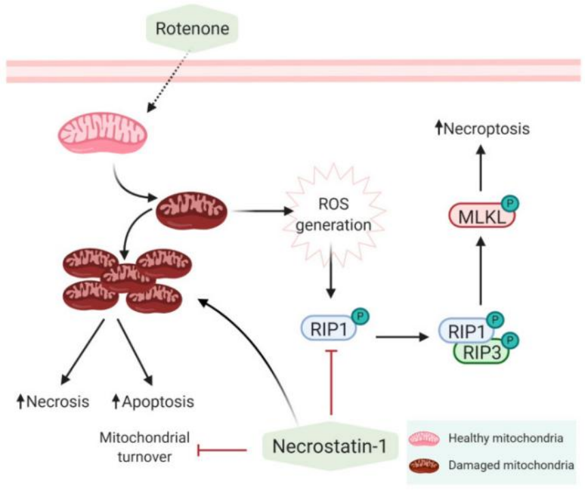
Fig 1. Mechanisms of action of rotenone and necrostatin-1. [5]
3.In an Alzheimer's disease (AD) model, Necrostatin-1 reduces necrosis and apoptosis in the cortex of aged APP/PS1 mice by decreasing the levels of phosphorylated RIPK3 and Bax, while increasing the level of Bcl-2.
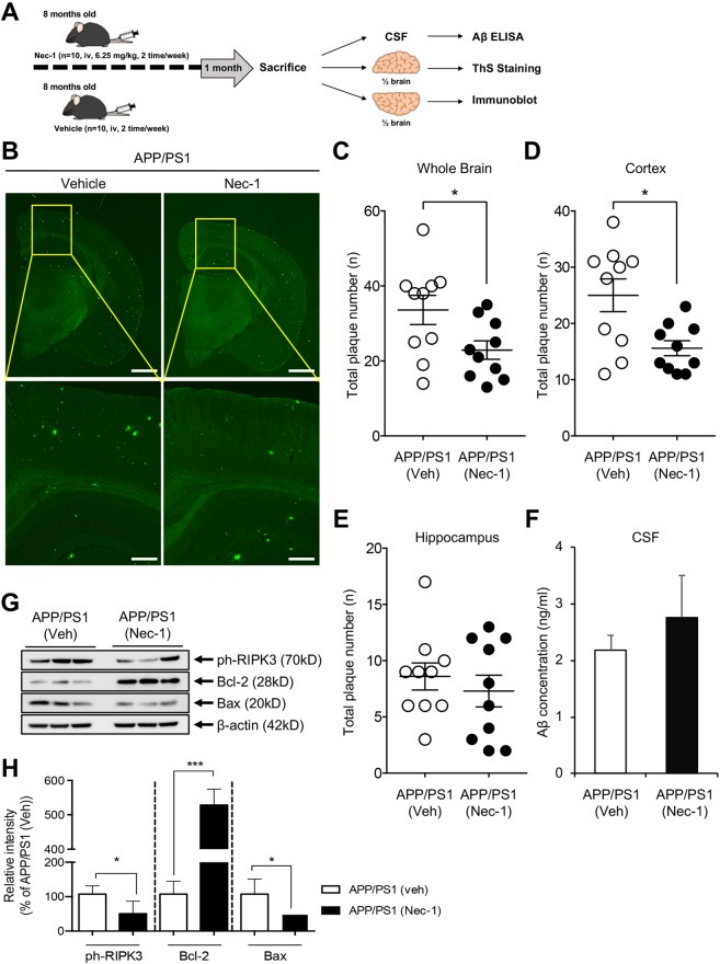
Fig 2.Nec-1 reduces Aβ plaques in aged APP/PS1 mouse brains.[6]
-Research onInflammatory Diseases
Programmed necrosis can trigger a strong inflammatory response, as the contents released after cell death can activate the immune system. For example, LPS and TNF-α can induce necrosis in macrophages, thereby promoting inflammation. At the same time, pathogens and their metabolic products, such as LPS, IL-1, and IL-6, can inhibit neutrophil apoptosis. Necrostatin-1 alleviates inflammation by inhibiting macrophage necrosis on one hand, and by inducing neutrophil apoptosis (through downregulation of McL-1 expression and upregulation of Bax) on the other, thus reducing inflammation[7].
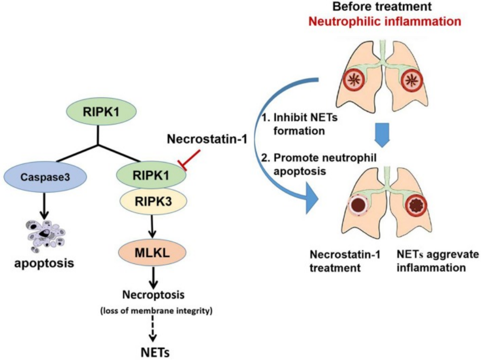
Fig 3. Schematic illustration of Necrostatin-1(Nec-1) ameliorates neutrophilic inflammation by suppressing MLKL phosphorylation to inhibiting NETs release. [8]
-Cancer Research
In certain types of cancer, programmed necrosis may play a dual role in the growth and spread of tumor cells. On the one hand, the activation of programmed necrosis can inhibit tumor growth; on the other hand, the inflammatory factors released by necrotic cell death may promote tumor invasion and metastasis. Studies have found that Necrostatin-1 can influence tumor development by regulating programmed necrosis, offering new insights for cancer treatment.
Overall, Necrostatin-1, as an inhibitor of programmed necrosis, has shown potential applications in various fields, including neurological diseases, inflammatory diseases, and cancer. Although its clinical application still faces challenges, with further research and the development of new inhibitors, Necrostatin-1 is expected to become an effective tool for treating multiple serious diseases in the future.
References
[1]Lin SS, Chang TM, Wei AI, Lee CW, Lin ZC, Chiang YC, Chi MC, Liu JF. Acetylshikonin induces necroptosis via the RIPK1/RIPK3-dependent pathway in lung cancer. Aging (Albany NY). 2023 Dec 19;15(24):14900-14914.
[2]Yuk H, Abdullah M, Kim DH, Lee H, Lee SJ. Necrostatin-1 Prevents Ferroptosis in a RIPK1- and IDO-Independent Manner in Hepatocellular Carcinoma. Antioxidants (Basel). 2021 Aug 25;10(9):1347.
[3]Cho YS. Perspectives on the therapeutic modulation of an alternative cell death, programmed necrosis (review). Int J Mol Med. 2014 Jun;33(6):1401-6.
[4]Deng XX, Li SS, Sun FY. Necrostatin-1 Prevents Necroptosis in Brains after Ischemic Stroke via Inhibition of RIPK1-Mediated RIPK3/MLKL Signaling. Aging Dis. 2019 Aug 1;10(4):807-817.
[5]Alegre-Cortés E, Muriel-González A, Canales-Cortés S, Uribe-Carretero E, Martínez-Chacón G, Aiastui A, López de Munain A, Niso-Santano M, Gonzalez-Polo RA, Fuentes JM, Yakhine-Diop SMS. Toxicity of Necrostatin-1 in Parkinson's Disease Models. Antioxidants (Basel). 2020 Jun 15;9(6):524.
[6]Yang SH, Shin J, Shin NN, Hwang JH, Hong SC, Park K, Lee JW, Lee S, Baek S, Kim K, Cho I, Kim Y. A small molecule Nec-1 directly induces amyloid clearance in the brains of aged APP/PS1 mice. Sci Rep. 2019 Mar 12;9(1):4183.
[7]Jie H, He Y, Huang X, Zhou Q, Han Y, Li X, Bai Y, Sun E. Necrostatin-1 enhances the resolution of inflammation by specifically inducing neutrophil apoptosis. Oncotarget. 2016 Apr 12;7(15):19367-81.
[8]Han XA, Jie HY, Wang JH, Zhang XM, Wang J, Yu CX, Zhang JL, He J, Chen JQ, Lai KF, Sun EW. Necrostatin-1 Ameliorates Neutrophilic Inflammation in Asthma by Suppressing MLKL Phosphorylation to Inhibiting NETs Release. Front Immunol. 2020 Apr 24;11:666.
[9]Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005 Jul;1(2):112-9. doi: 10.1038/nchembio711. Epub 2005 May 29. Erratum in: Nat Chem Biol. 2005 Sep;1(4):234.
Other Articles



Subscription to TargetMol News
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.