- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty

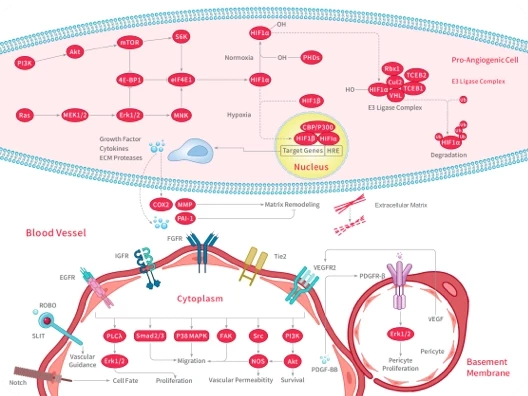
Biomolecular Interaction
Kd/Ka/KD analyzed. Binding affinity quantified.
Testing Methods
Effectiveness
Supply
Instrucments
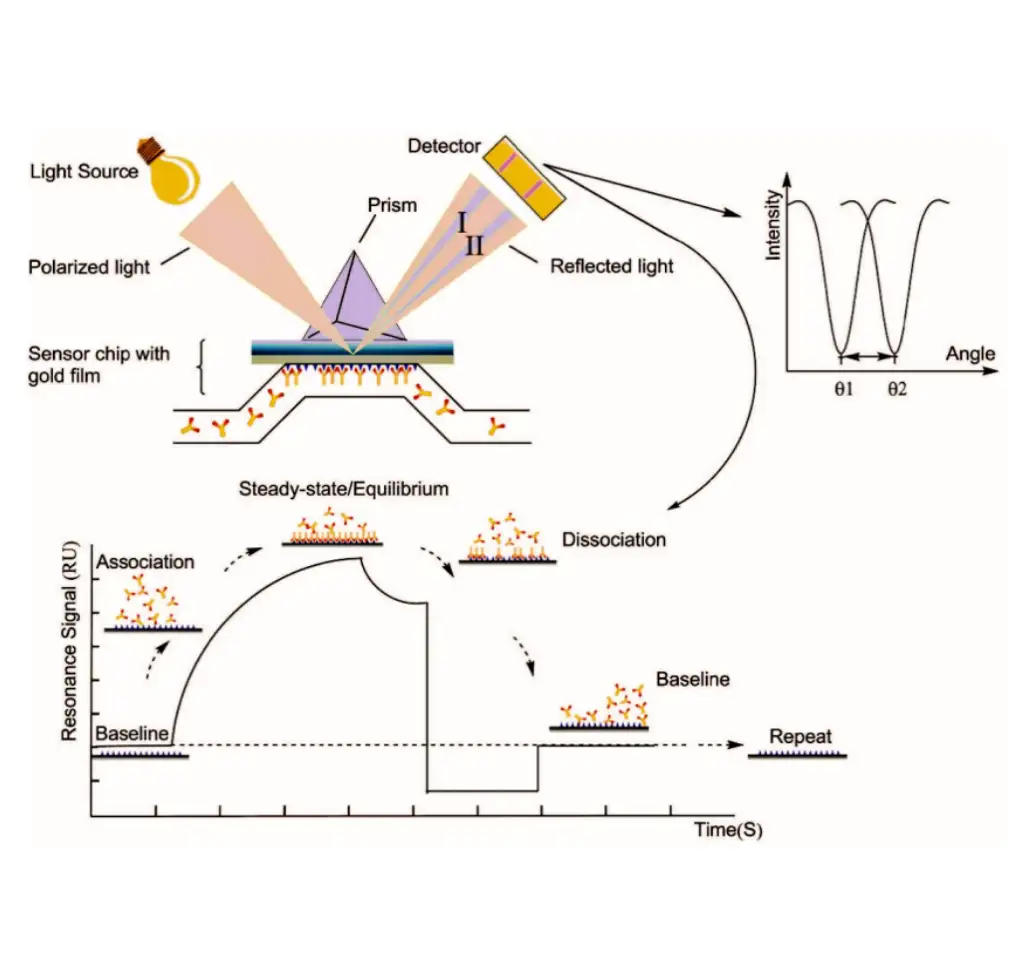
SURFACE PLASMON RESONANCE (SPR)
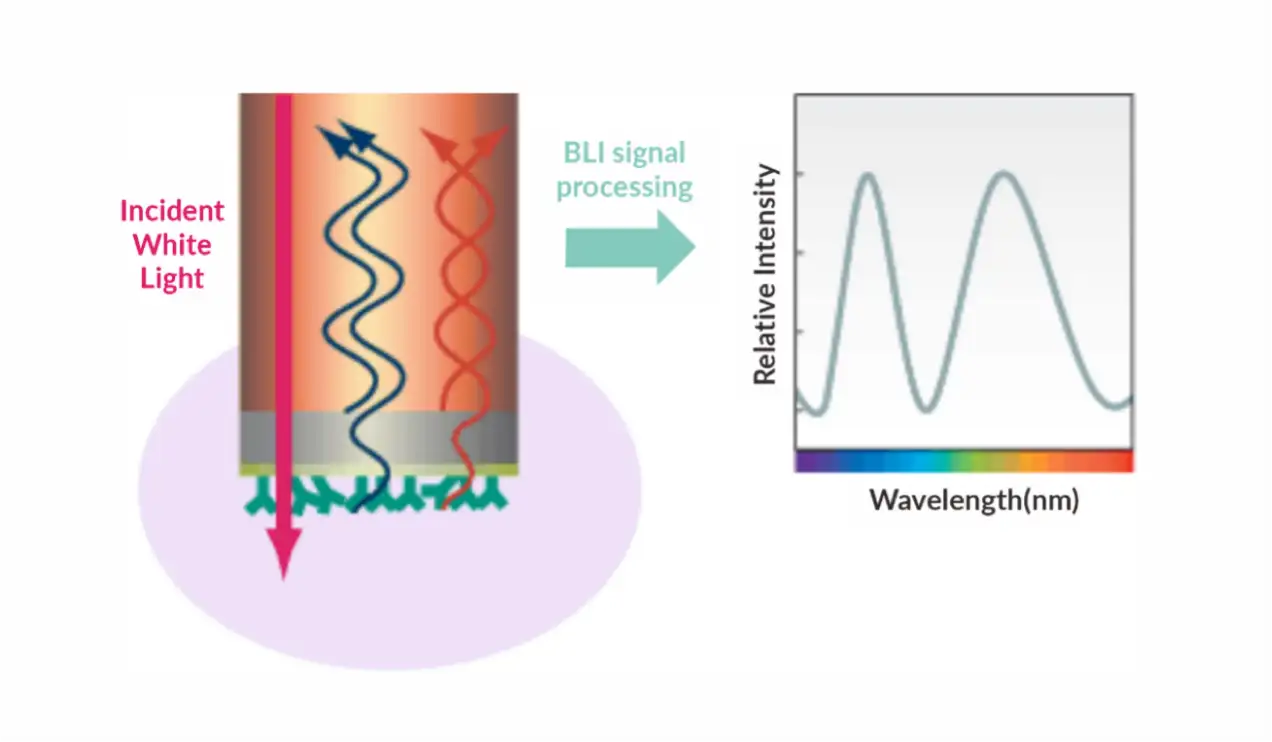
Principle: Surface Plasmon Resonance (SPR) is an optical phenomenon induced by either photons or electrons. When light is incident from an optically dense medium to an optically sparse medium, total internal reflection occurs, forming an evanescent wave entering the optically sparse medium. The incident angle causing Surface Plasmon Resonance is referred to as the SPR angle. SPR phenomena are related to the refractive index of the metal surface. When an analyte binds to the chip, refractive index alters that causing a change in the SPR angle. Biosensors detect these changes in real-time, allowing the monitoring of molecular interactions. In experiments, one biological molecule is immobilized on the surface of chip, and the molecule interacting with it is dissolved in a solution flowing over the chip surface. The detector tracks the changes in the binding and dissociation of molecules in real-time. Surface Plasmon Resonance (SPR) based on Biacore allow highly sensitive real-time monitoring of interaction between two molecules(including proteins, molecules, DNA, RNA), enabling high-throughput screening. As an innovative biosensing analysis technology, SPR experiments span every stage of drug development, including target discovery, drug screening, proteomics, immunogenicity, biopharmaceutical research and development, production, as well as life science research.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.